Kit and method for detecting procalcitonin
A procalcitonin and kit technology, applied in the field of immunoassays, can solve problems such as unstable markers, difficulty in ensuring repeatability of results, and limited internal surface area, so as to improve detection sensitivity and accuracy and avoid reduction in luminous efficiency , the effect of large antibody coating surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] 1. Preparation of magnetic particle-conjugated antibodies
[0037] The magnetic particle-coupled antibody in the kit is used as a stationary separation phase. The antibody in the magnetic particle-coupled antibody is procalcitonin monoclonal antibody. The concentration of the magnetic particle used for coupling is 1-10 mg / mL. The concentration of the linked antibody is 1-5mg / mL, the size of the magnetic particle is 200nm-5μm, and the active group on the surface of the magnetic particle is -COOH or -NH 2 , the magnetic particles are activated by chemical cross-linking reagents, so that they can be separated from the -NH of procalcitonin monoclonal antibody 2 Or the -COOH group is covalently bonded to form a stable magnetic particle conjugate. In this embodiment, the magnetic particle whose active group is -COOH is the first choice. Under the action of procalcitonin monoclonal antibody -NH 2 The group forms an amide bond and is covalently bonded. The magnetic particle-c...
Embodiment 2
[0059]The detection method of procalcitonin, the detection process can adopt a one-step method or a two-step method reaction mode: in the one-step method, the working solution, the serum to be tested and the magnetic particle-coupled antibody solution are added to the reaction tube at the same time, and after a certain period of reaction , magnetic separation and washing, adding a luminescent substrate, luminol or isoluminol emits photons under the catalysis of HRP, and the luminescent signal is detected by the detector. In the two-step method, the serum to be tested and the working solution are first added to the reaction tube to react for a certain period of time, then the magnetic particle-coupled antibody solution is added, after a certain period of time, the magnetic separation and washing are added, and the luminescent substrate, luminol or isoglucose, is added. Under the catalysis of Minol HRP, photons are emitted, and the luminescent signal is detected by the detector. ...
Embodiment 3
[0067] Embodiment three: the methodological evaluation of kit of the present invention
[0068] 1. Accuracy
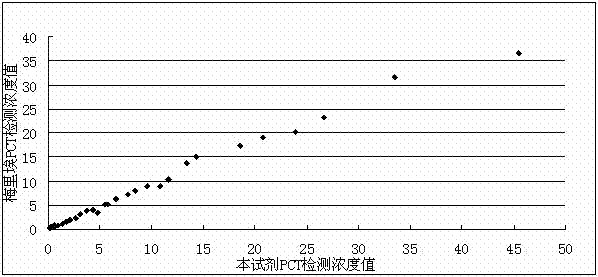
[0069] Accuracy measurement refers to adding a known amount of PCT standard substance to normal human serum samples, and comparing the measured concentration value with the added theoretical value to calculate the recovery rate of PCT. The test results are as follows:
[0070]
[0071] 2. Precision
[0072] Three specimens with different concentrations were selected, and the measurements were repeated 20 times according to the method of the present invention. According to the measurement results of 20 times, calculate the average deviation C.V.% value.
[0073]
[0074] 3. Analytical sensitivity
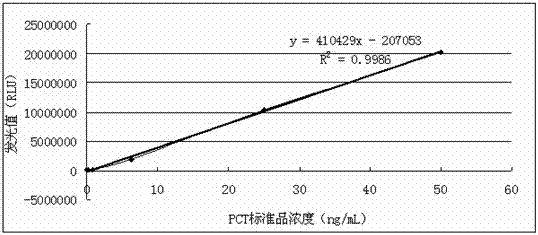
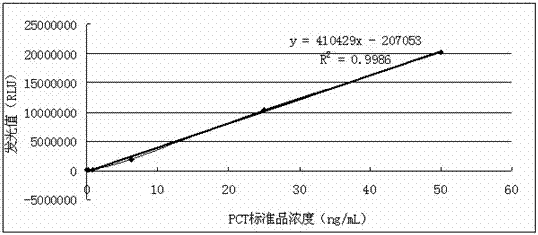
[0075] Analytical sensitivity is defined as: Refers to the quantity that can be distinguished from zero dose in a statistical sense. Repeat 20 times to measure the zero dose point, calculate the mean (X) and standard deviation (SD), and the calculated concentration...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Analytical sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com