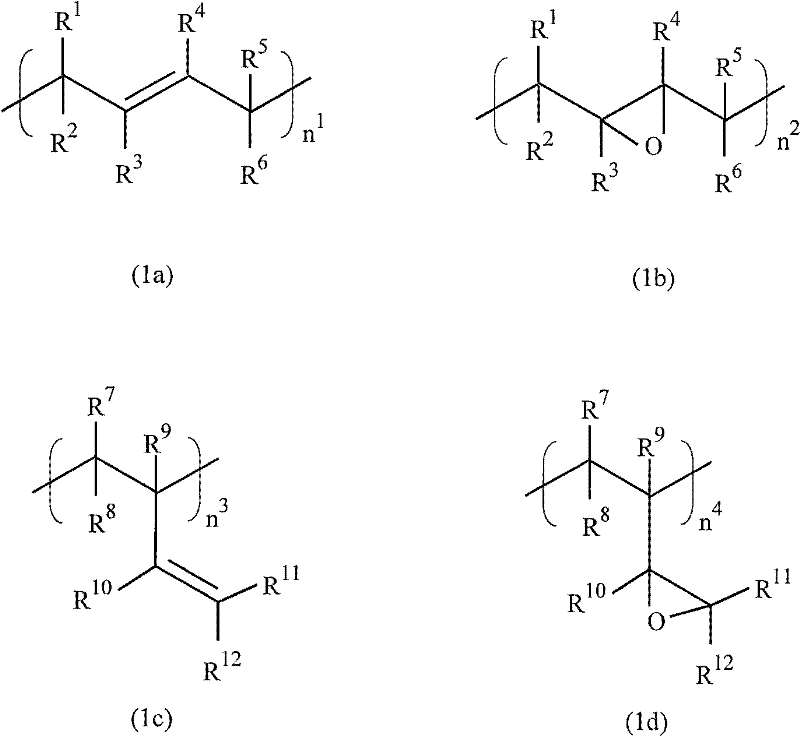
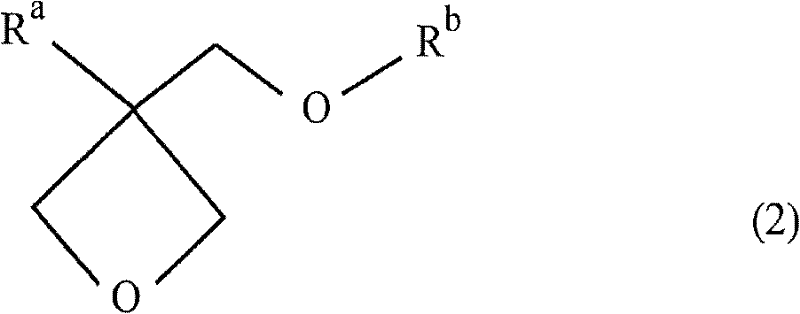
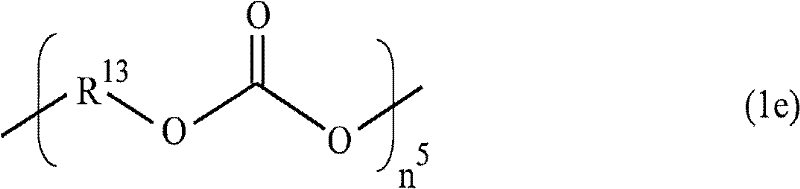Cation-polymerizable resin composition and cured product thereof
A cationic polymerization, resin composition technology, applied in other chemical processes, epoxy resin coatings, chemical instruments and methods, etc., can solve the problems of heat resistance, poor transparency, difficult application, lack of flexibility, etc., to achieve easy processing. , Excellent workability, excellent safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0202] Raise the temperature of 280 mL of a mixture of 24.9 g (0.23 mol) of sodium carbonate and toluene to 95° C., add 1.4 g of propionic acid, and dropwise add 16 g of vinyl acetate while keeping the temperature at 95° C. After 15 minutes, add di-μ- Chlorobis(1,5-cyclooctadiene)diiridium(|)[Ir(cod)Cl] 2 1.27g (1.9mmol). Next, 40 g (0.19 mol) of oxetane-3,3-dimethanol was added dropwise over 3 hours, and 79.8 g of vinyl acetate was added dropwise while maintaining the temperature at 95° C. under a nitrogen atmosphere to perform a reaction. After the dropwise addition was completed, stir for 1 hour, and analyze the reaction solution by gas chromatography, 3,3-bis(vinyloxymethyl)oxetane represented by the following formula (13) was generated with a yield of 90%. , (3-vinyloxymethyloxetan-3-yl)methanol was produced in 2% yield. The reaction solution was purified by distillation to obtain 31 g of 3,3-bis(vinyloxymethyl)oxetane with a purity of 99%.
[0203] 1 H-NMR (CDCl 3 )...
Synthetic example 2
[0206] 3-chloromethyl-3 ethyl oxetane (0.1mol) and 1,4-cyclohexanediol (0.5mol), tetrabutylammonium bromide (0.01mol) were added in toluene (500g), After heating up to 90°C, 5N-NaOH aqueous solution (100 g) was added dropwise, followed by stirring for 5 hours. The toluene solution (toluene layer) was washed with water, concentrated, and purified by silica gel chromatography to obtain 4-(3-ethyl-oxetan-3-yl-methoxy)cyclohexanol with a purity of 99%.
[0207] 100 mL of a mixture of sodium carbonate (0.06 mol) and toluene was heated to 95°C. While keeping the temperature at 95°C, 4.2 g of vinyl acetate was added dropwise, and after 15 minutes, di-μ-chlorobis(1,5-cyclooctadiene)diiridium(|)[Ir(cod)Cl] was added 2 (0.5 mmol). Next, 4-(3-ethyloxetan-3-yl-methoxy)cyclohexanol (0.05 mmol) was added dropwise over 2 hours, under a nitrogen atmosphere while maintaining the temperature at 95°C, While adding 12.6 g of vinyl acetate dropwise, the reaction was carried out. After the drop...
Synthetic example 3
[0210] 12.6 g (0.1 mol) of (4-methylcyclohex-3-enyl)methanol was epoxidized at 65° C. using a 5% by weight peracetic acid-ethyl acetate solution. Purification by distillation yielded 12 g of (6-methyl-7-oxabicyclo[4.1.0]hept-3-yl)methanol with a purity of 98%.
[0211] 100 mL of a mixture of sodium carbonate (0.06 mol) and toluene was heated to 95°C. While keeping the temperature at 95°C, 4.2 g of vinyl acetate was added dropwise, and after 15 minutes, di-μ-chlorobis(1,5-cyclooctadiene)diiridium(|)[Ir(cod)Cl] was added 2 (0.5 mmol). Next, (6-methyl-7-oxabicyclo[4.1.0]hept-3-yl)methanol (0.05 mmol) was added dropwise over 2 hours, and the temperature was kept at 95°C under a nitrogen atmosphere. 12.6 g of vinyl acetate was dripped and reacted. After the dropwise addition was completed, stir for 1 hour, analyze the reaction solution by gas chromatography, and generate 1-methyl-4-vinyloxy-7-oxabicyclo[ 4.1.0] Heptane. determine its 1 H-NMR (CDCl 3 ), as in Synthesis Exampl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com