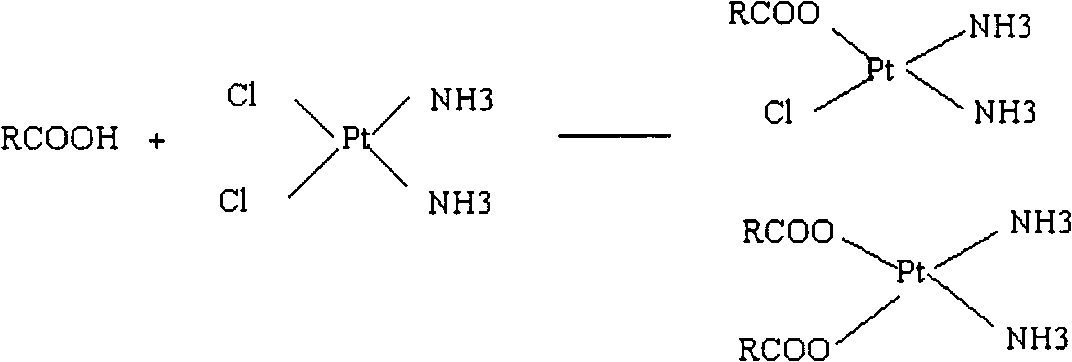
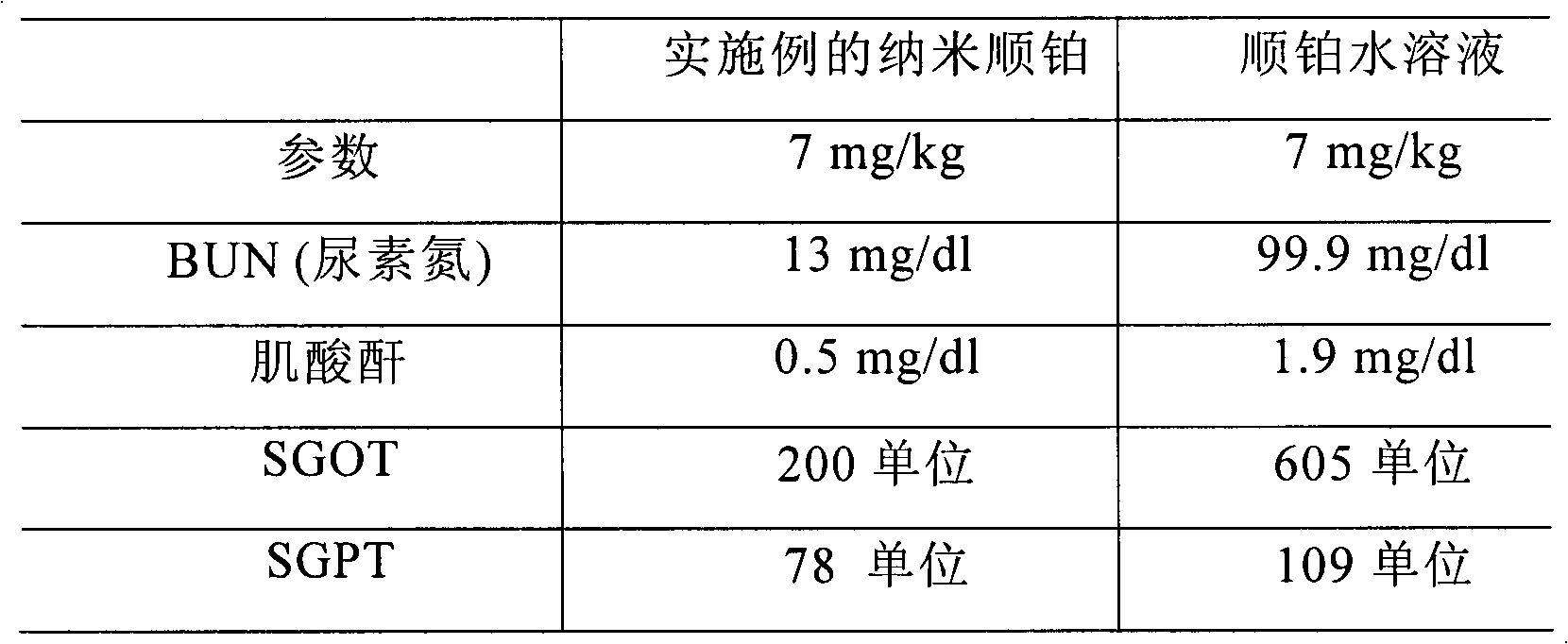
Novel cis-platinum nano biodegradable medicinal composition and preparation method
A cisplatin and nanoparticle technology, applied in the field of medical treatment, can solve the problems of cisplatin difficult to embed, cisplatin cannot be retained, and low water solubility, and achieve the effect of simplified preparation method and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] In this embodiment, the main operation is to dissolve the injectable sodium carboxymethylcellulose polymer complying with FDA specifications in water with sterile demineralized water, and the prepared concentration is 1.3%; then add cisplatin (0.6%) to the solution Dissolve in medium, react at 37°C for several hours, and form a complex compound derived from the copolymer and cisplatin represented by the following formula; the equivalent ratio of the Pt atom of cisplatin to the carboxyl anion of the copolymer (Pt / COO - ) is 0.3 or more. In this example, cisplatin, which is poorly soluble in water when in unconjugated form, exhibited a greatly improved solubility after conjugation.
[0052] Chemical reaction process:
[0053]
[0054] Sodium Carboxymethyl Cellulose Molecular Structure I:
[0055] The complex described in the present invention means that one or both of the two chloride ions in the CDDP molecule are exchanged by the carboxyl anion of the copolymer to...
Embodiment 2
[0057] 600.1mg of PLGA-mPEG two-block copolymer, the two-block molecular weight is 60,000 and 5,000 respectively, dissolved in 20mL of dichloromethane / acetone, 937.2mg of Span 80 was dissolved in the composite organic phase, the organic phase solution and cisplatin complex Mix the mixture solution to form colostrum; add the colostrum to 100mL 0.3% polyethylene glycol vitamin E succinate solution, and then put the uniformly dispersed mixture solution under high pressure (9,000~16,000psi) in a micro jet homogenizer Internal (suitable sterilization) treatment until a nanoemulsion is obtained (particle size less than 0.2 micron); volatilize for 2-6 hours and pass the submicron emulsion through a 0.2 micron filter to sterilize the emulsion. It was frozen to -80°C under aseptic conditions and lyophilized for 58 hours while warming to 30°C.
[0058]The resulting powder containing 5.7% (w / w) cisplatin was reconstituted with 0.9% aqueous sodium chloride solution to form a cisplatin con...
Embodiment 3
[0060] 600.3mg of PLGA-mPEG two-block copolymer, the two-block molecular weight is 30,000 and 5,000 respectively, dissolved in 20mL of dichloromethane / acetone, 937.5mg of Span 80 was dissolved in the composite organic phase, the organic phase solution and cisplatin complex Mix the mixture solution to form colostrum; add the colostrum to 100mL 0.3% polyethylene glycol vitamin E succinate solution, and then put the uniformly dispersed mixture solution under high pressure (9,000~16,000psi) in a micro jet homogenizer Internal (suitable sterilization) treatment until a nanoemulsion is obtained (particle size less than 0.2 micron); volatilize for 2-6 hours and pass the submicron emulsion through a 0.2 micron filter to sterilize the emulsion. It was frozen to -80°C under aseptic conditions and lyophilized for 58 hours while warming to 30°C.
[0061] The resulting powder containing 5.7% (w / w) cisplatin particles was reconstituted with 0.9% aqueous sodium chloride to form a cisplatin c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com