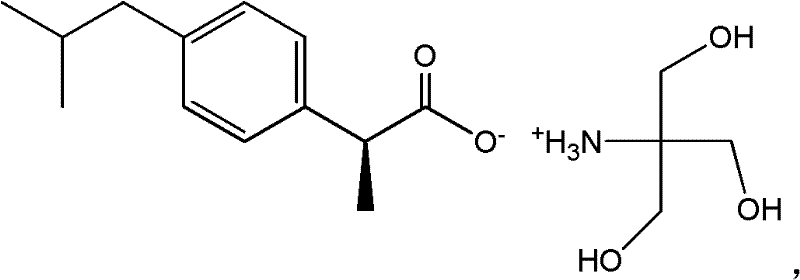
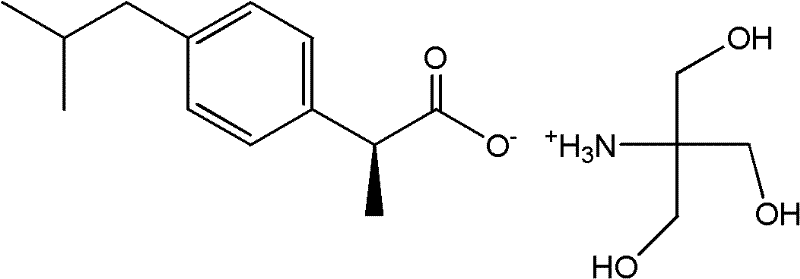
Preparation methods of S-(+)-ibuprofen tromethamine salt and oral solution thereof
A technology for dexibuprofen and oral solution, which is applied in the preparation of carboxylate salts, organic compounds, amino hydroxyl compounds, etc., can solve the problems of low water solubility of dexibuprofen, and achieve accurate dosage and good taste Improvement, water-solubility enhancement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Preparation of dextroibuprofen tromethamine salt
[0045] Accurately weigh 2.422g of tromethamine and dissolve it in 25mL of distilled water, heat to 50℃ to dissolve, and accurately weigh 4.130g of dextro-ibuprofen (the molar ratio of tromethamine is 1:1) in a mill Add 17 mL of 95% ethanol to a round-bottomed bottle. After stirring to dissolve, slowly add the above tromethamine aqueous solution, stirring at room temperature until white precipitation appears, and the solvent is evaporated to obtain the crude product. Recrystallized with 95% ethanol, the yield is 94.6 %. The measured melting point is 158~162℃, and the water solubility is 5.0~6.0mg / mL.
[0046] 1 HNMR (ppm): 0.876 (d, H a ), 1.296(s, H b ), 1.798(m, H c ), 3.390(s, H d ), 5.816(m, H e ), 7.101(q, H e ), 2.428(t, H f )
[0047] IR(kBr)(cm -1 ): 1183.77cm -1 It is the stretching vibration of dextroibuprofen carboxyl group, 1403.29cm -1 And 1,631.73cm -1 It is the characteristic band of carboxyl ion ban...
Embodiment 2
[0048] Example 2: Preparation of dextroibuprofen tromethamine salt
[0049] Accurately weigh 2.450g of tromethamine and dissolve it in 50mL of distilled water, heat to 40℃ to dissolve, and accurately weigh 8.315g of dextro-ibuprofen (the molar ratio of tromethamine is 2:1) in a mill Add 30 mL of 95% ethanol to a round-bottomed flask. After stirring to dissolve, the tromethamine aqueous solution is slowly added dropwise and stirred at room temperature until white precipitation appears. The solvent is evaporated to obtain the crude product, which is recrystallized with acetone. The yield is 61.73%. The measured melting point is 158~162℃, and the water solubility is 5.0~6.0mg / mL.
[0050] 1 HNMR (ppm): 0.876 (d, H a ), 1.296(s, H b ), 1.798(m, H c ), 3.390(s, H d ), 5.816(m, H e ), 7.101(q, H e ), 2.428(t, H f )
[0051] IR(kBr)(cm -1 ): 1183.77cm -1 It is the stretching vibration of dextroibuprofen carboxyl group, 1403.29cm -1 And 1,631.73cm -1 It is the characteristic band of carboxy...
Embodiment 3
[0052] Example 3: Preparation of dextroibuprofen tromethamine salt
[0053] Accurately weigh 2.320g of tromethamine and dissolve in 50mL of distilled water, heat to 60℃ to dissolve, and accurately weigh 2.006g of dextro-ibuprofen (molar ratio of tromethamine is 1:2) in a mill Add 30 mL of 95% ethanol to a round-bottomed flask. After stirring to dissolve, slowly add dropwise the above tromethamine aqueous solution, stir at room temperature until white precipitation appears, distill off the solvent to obtain a crude product, and recrystallize with methanol. The yield is 70.65%. The measured melting point is 158~162℃, and the water solubility is 5.0~6.0mg / mL.
[0054] 1 HNMR (ppm): 0.876 (d, H a ), 1.296(s, H b ), 1.798(m, H c ), 3.390(s, H d ), 5.816(m, H e ), 7.101(q, H e ), 2.428(t, H f )
[0055] IR(kBr)(cm -1 ): 1183.77cm -1 It is the stretching vibration of dextroibuprofen carboxyl group, 1403.29cm -1 And 1,631.73cm -1 It is the characteristic band of carboxyl ion band, 714.69cm ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Water soluble | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com