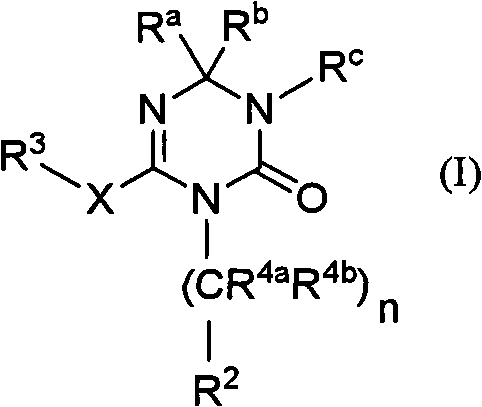
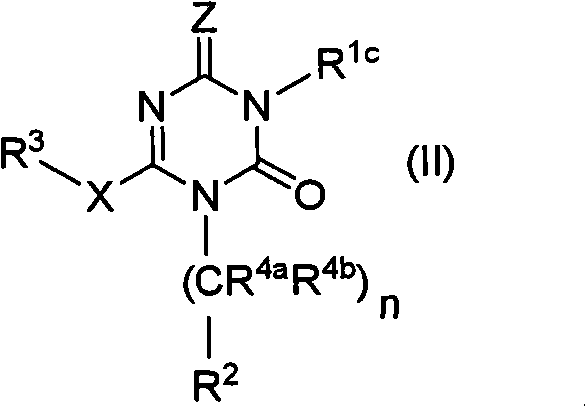
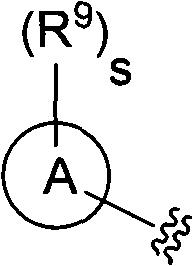
Novel triazine derivative and pharmaceutical composition containing same
A compound and solvate technology, applied in the field of novel triazine derivatives and pharmaceutical compositions containing the triazine derivatives, can solve the problem of unrecorded analgesic receptor antagonism, unrecorded receptor antagonism, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0743] Preparation of 6-(ethylthio)-1-(4-fluorobenzyl)-3-isopropyl-1,3,5-triazine-2,4(1H,3H)-dione
[0744] [Chemical formula 62]
[0745]
[0746] To the mixture of S-ethylthiourea hydrobromide (14.8g, 80mmol) and DMF (75mL), add isopropyl isocyanate (8.2mL, 84mmol) and DBU (12.6mL, 84mmol) under ice cooling, Stir for 6 hours under ice cooling. To the reaction solution, 1,1'-carbonyldiimidazole (15.57 g, 96 mmol) and DBU (18.0 mL, 120 mmol) were added under ice cooling, and the mixture was further stirred for 2 hours. After the reaction, 2 mol / L hydrochloric acid (240 mL) was added over about 50 minutes under ice cooling, and the precipitated solid was collected by filtration. The obtained solid was dissolved in ethyl acetate, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain 6-(ethylthio)-3-isopropyl-1,3,5- as a light brown solid. Triazine-2,4(1H,3H)-dione (12.29g, yield: 71%).
[0747] 1H-NMR (δppm TMS / DMSO-d6): 1.27 (6H, t, J=7.2 Hz)), ...
Embodiment 2
[0751] 1-(4-Fluorobenzyl)-6-(4-isopropoxyphenylamino)-3-isopropyl-1,3,5-triazine-2,4(1H,3H)-dione Preparation
[0752] [Chemical formula 63]
[0753]
[0754] The 6-(ethylthio)-1-(4-fluorobenzyl)-3-isopropyl-1,3,5-triazine-2,4(1H,3H)-dione (0.323g, 1mmol ), 4-isopropoxyaniline (0.907 g, 6 mmol), and 1-methyl-2-pyrrolidone (1 mL), and stirred at 230°C for 30 minutes under microwave irradiation. After the reaction, water (100 mL) was added, and extraction was performed with ethyl acetate (100 mL). After the extract was dried over anhydrous sodium sulfate, and concentrated under reduced pressure, the obtained residue was purified by silica gel column chromatography (ethyl acetate / hexane). The obtained target product was precipitated in ethyl acetate and hexane to obtain 1-(4-fluorobenzyl)-6-(4-isopropoxyphenylamino)-3-isopropyl as a colorless solid -1,3,5-triazine-2,4(1H,3H)-dione (0.21g, yield: 51%).
[0755] Melting point: 176-177°C
[0756] 1H-NMR(δppm TMS / CDCl 3 ): 1.34 (6H, d, ...
Embodiment 3
[0758] 6-(3-Chloro-4-isopropoxyphenylamino)-1-(4-chlorobenzyl)-3-isopropyl-1,3,5-triazine-2,4(1H,3H )-Diketone preparation
[0759] [Chemical formula 64]
[0760]
[0761] The 1-(4-chlorobenzyl)-6-(ethylthio)-3-isopropyl-1,3,5-triazine-2,4(1H,3H)-dione (0.60g, 1.78 mmol), 3-chloro-4-isopropoxyaniline (0.99 g, 5.3 mmol) and acetic acid (10 mL) were stirred at 90°C for 6 hours. After the reaction, the reaction solution was added to a saturated aqueous sodium hydrogen carbonate solution (100 mL), and extracted with ethyl acetate (100 mL). The extract was washed with saturated aqueous sodium hydrogen carbonate (100 mL) and saturated brine (100 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The obtained residue was purified by silica gel column chromatography (ethyl acetate / hexane). The obtained target product was precipitated in diethyl ether and hexane to obtain 6-(3-chloro-4-isopropoxyphenylamino)-1-(4-chlorobenzyl)-3 as a colorless solid -Isop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com