Method for preparing shell-sheddable polymer micelle drug carrier
A technology of polymer glue and hydrophilic polymer, which is applied in the field of preparation of polymer micellar drug carriers, and can solve the problems of few research reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The invention provides a novel PEG shell bridged by disulfide bonds, which can be shed and loaded with tumor drugs. It is characterized in that under the condition of glutathione reduction in tumor cells, the disulfide bond is broken, and the drug can be released rapidly to achieve the controlled release of the drug to the tumor cells, forming a new type of high-efficiency tumor drug carrier.
[0029] Aminated methoxypolyethylene glycol (mPEG-SS-NH 2 ) is a hydrophilic polymer, β-benzyloxycarbonyl protected polylysine (PzLL) is a hydrophobic polymer, and doxorubicin (DOX) is used as a tumor drug model, and the preparation, function and effect of the drug carrier of the present invention are made. Comprehensive and detailed introduction:
[0030] (1) Acyl chloride of dithiodipropionic acid: Under nitrogen protection, take dithiodipropionic acid (DTDP), 1.05g, 5mmol and dissolve it in 5mL thionyl chloride (SOCl 2 ) solution, reacted at 85°C for 4h, then spin-dried SOCl ...
Embodiment 2
[0036] Preparation of shell exfoliated polymer micelles and determination of the lowest critical micelle concentration
[0037] Amphiphilic polymer micelles were prepared according to the method of Example 1, and then the micelles were prepared and the minimum critical micelle concentration (CMC) was determined.
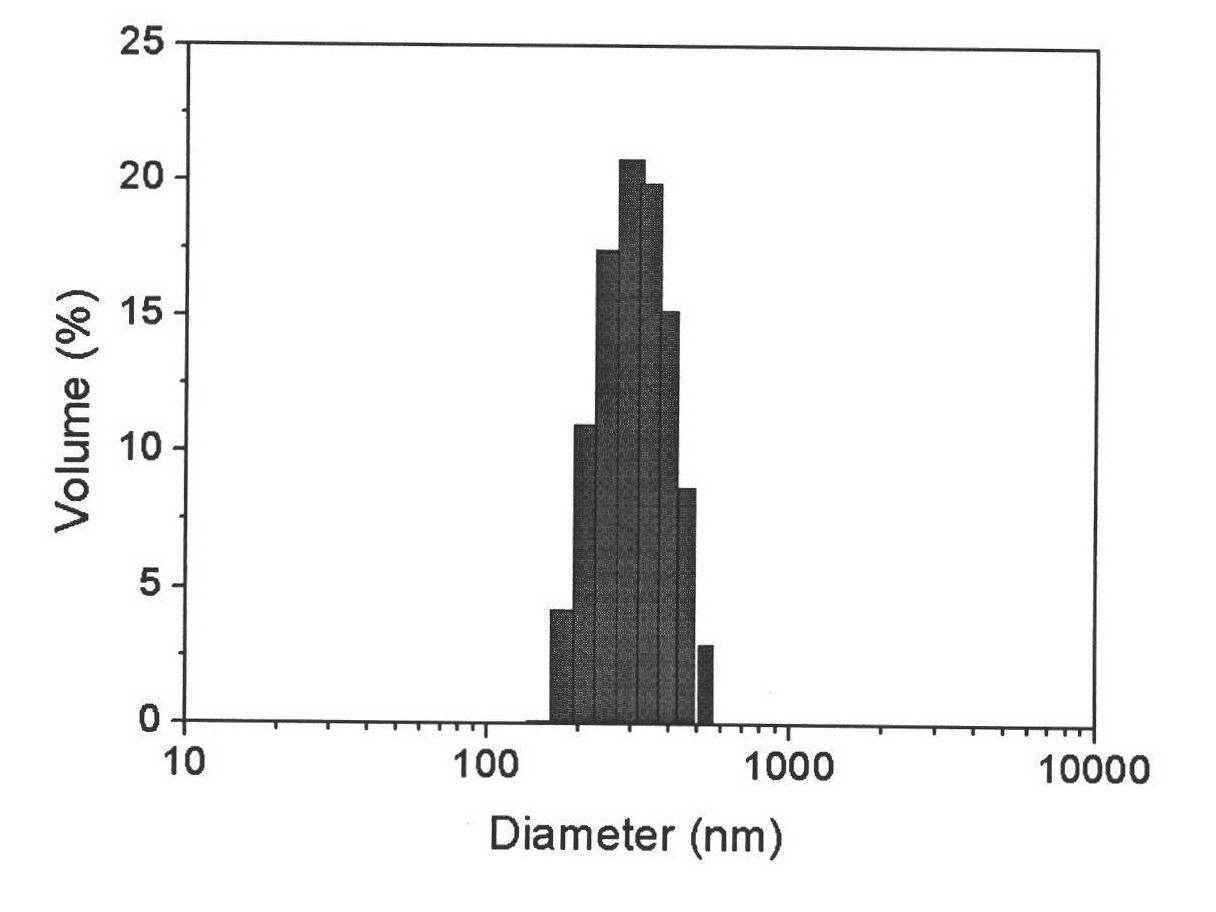
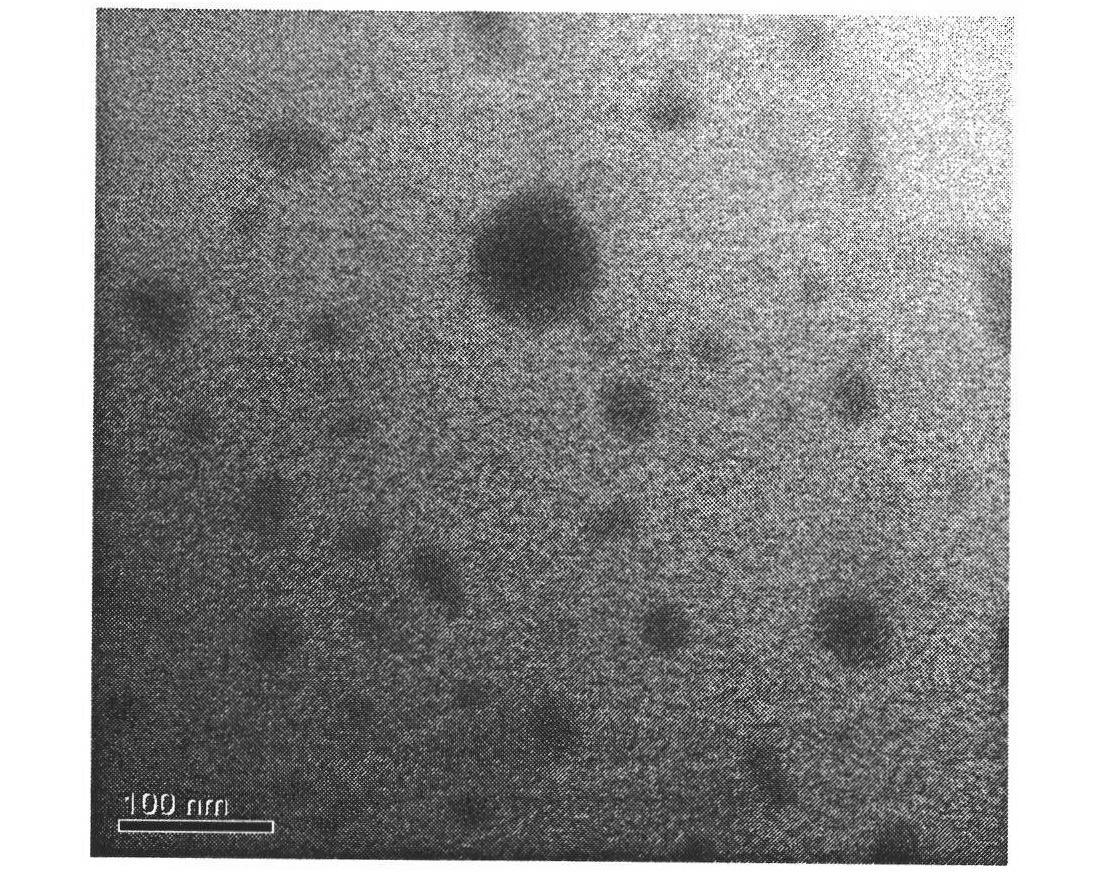
[0038] The mPEG-SS-PzLL polymer micelles are obtained by dialysis, and the particle size is measured by dynamic light scattering (DLS). The particle size is about 302nm, the particle size distribution is narrow, and the PDI is 0.048±0.005, such as figure 1 As shown, the micelles are in a spherical shape, and the electron microscope pictures are as follows figure 2 shown.
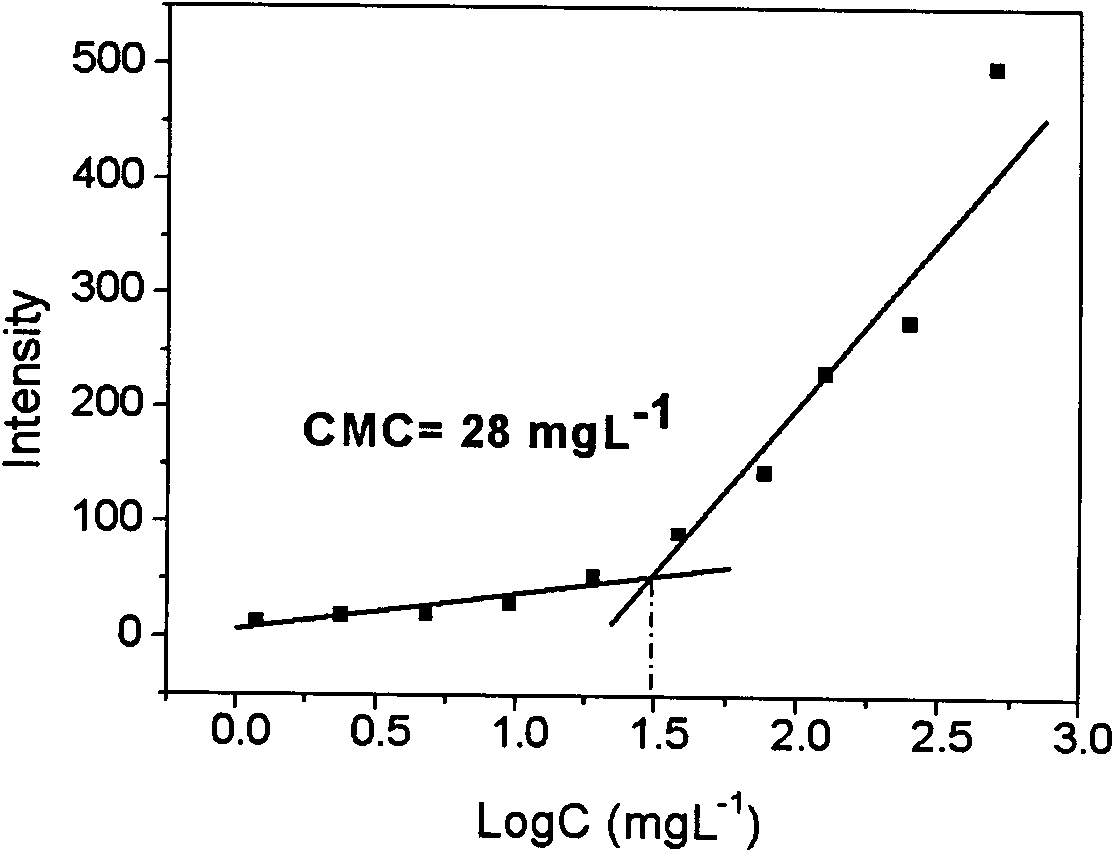
[0039] The minimum critical micelle concentration (CMC) of the micelles was determined by encapsulating the hydrophobic probe fluorescent pyrene. To ten 10mL centrifuge tubes, add 1mL concentration of 6×10 -6 The acetone solution of mol / L pyrene was placed in a centrifuge tube, and after the ac...
Embodiment 3
[0041] Glutathione stimulation-induced shedding of micellar shell
[0042] The amphiphilic polymer micelles were prepared according to the method in Example 2, and then the micelles shell exfoliation induced by glutathione (GSH) stimulation was studied.
[0043] Micellar shell shedding properties were illustrated by measuring the change in particle size of polymeric micelles using DLS in a 10 mM GSH environment. After adding 10mM GSH for 2h, the particle size of the polymer increased from 300nm to 419nm, and the PDI increased from 0.048 to 0.22, from Figure 5 shown. When the time was increased to 4h, a large aggregation occurred, which was due to the breaking of the disulfide bond, which caused the departure of the hydrophilic PEG, resulting in the aggregation of the hydrophobic end. Thus it is proved that under the environment of glutathione, the micellar shell layer can be shed and the drug can be released.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com