Method for preparing moxifloxacin hydrochloride intermediate
A technology for moxifloxacin hydrochloride and intermediates, which is applied in the field of preparation of moxifloxacin hydrochloride intermediates, can solve the problems of many steps, low yield, cumbersome operation, etc., and achieve the effect of fewer steps, high yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
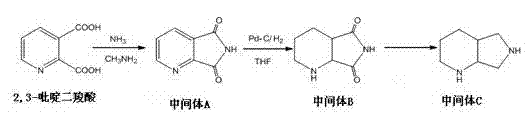
[0020] Add 200ml of methylamine into a 500ml three-neck flask, add 80g of 2,3-pyridinedicarboxylic acid while stirring, heat and dissolve slowly until the reaction is refluxed for 48h, TLC detects that the reaction of 2,3-pyridinedicarboxylic acid is complete, cool to room temperature, Slowly poured into ice water, a large amount of solids precipitated while stirring, extracted 3 times with dichloromethane, washed the organic layer with water once, and anhydrous Na 2 SO 4 Drying and concentrating under reduced pressure to dryness yielded 50 g of yellow oil intermediate A with a yield of 75%.
[0021] Dissolve 50g of intermediate A in 375ml of methanol, stir, add 10g of 5% Pd-C, and catalyze hydrogenation in a 1L autoclave at 3Mpa and 65°C for 4h. TLC detects that the reaction of intermediate A is complete, stop heating, and filter out Pd- C, concentrated to dryness under reduced pressure to obtain 45g oil intermediate B.
[0022] Add THF0.45L into a 1L three-neck flask, cool...
Embodiment 2
[0024] Add 550ml of methylamine into a 1L three-necked flask, add 220g of 2,3-pyridinedicarboxylic acid while stirring, heat and dissolve slowly until the reflux reaction is 50h, TLC detects that the reaction of 2,3-pyridinedicarboxylic acid is complete, cool to room temperature, Slowly poured into ice water, a large amount of solids precipitated while stirring, extracted 3 times with dichloromethane, washed the organic layer with water once, and anhydrous Na 2 SO 4 Drying and concentrating under reduced pressure to dryness gave 200 g of yellow oil intermediate A with a yield of 80%.
[0025] Dissolve 200g of intermediate A in 1500ml of methanol, stir, add 40g of 5% Pd-C, and catalyze hydrogenation in a 1L autoclave at 3Mpa, 65°C for 6h. TLC detects that the reaction of intermediate A is complete, stop heating, and filter out Pd- C, concentrated to dryness under reduced pressure to obtain 190g of oil intermediate B.
[0026] Add THF1.9L into a 3L three-neck flask, cool in an...
Embodiment 3
[0028] Add 950ml of methylamine into a 2L three-necked flask, add 380g of 2,3-pyridinedicarboxylic acid while stirring, heat and slowly dissolve until reflux reaction for 48h, TLC detects that the reaction of 2,3-pyridinedicarboxylic acid is complete, cool to room temperature, Slowly poured into ice water, a large amount of solids precipitated while stirring, extracted 3 times with dichloromethane, washed the organic layer with water once, and anhydrous Na 2 SO 4 Drying and concentrating under reduced pressure to dryness gave 362g of yellow oil intermediate A with a yield of 82%.
[0029] Dissolve 362g of intermediate A in 2715ml of methanol, stir, add 72g of 5% Pd-C, and catalyze hydrogenation in a 1L autoclave at 3Mpa, 65°C for 6h. TLC detects that the reaction of intermediate A is complete, stop heating, and filter out Pd- C, concentrated to dryness under reduced pressure to obtain 350g oil intermediate B.
[0030] Add 3.5L THF to a 5L three-neck flask, cool in an ice bat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com