Method of decomposing hydrogen sulfide for preparation of hydrogen and elemental sulfur
A technology for hydrogen sulfide and elemental sulfur, applied in the preparation/purification of sulfur, hydrogen production, etc., can solve the problems of harsh separation operation, difficult to complete separation, impossible to achieve complete conversion, etc., and achieve universal effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
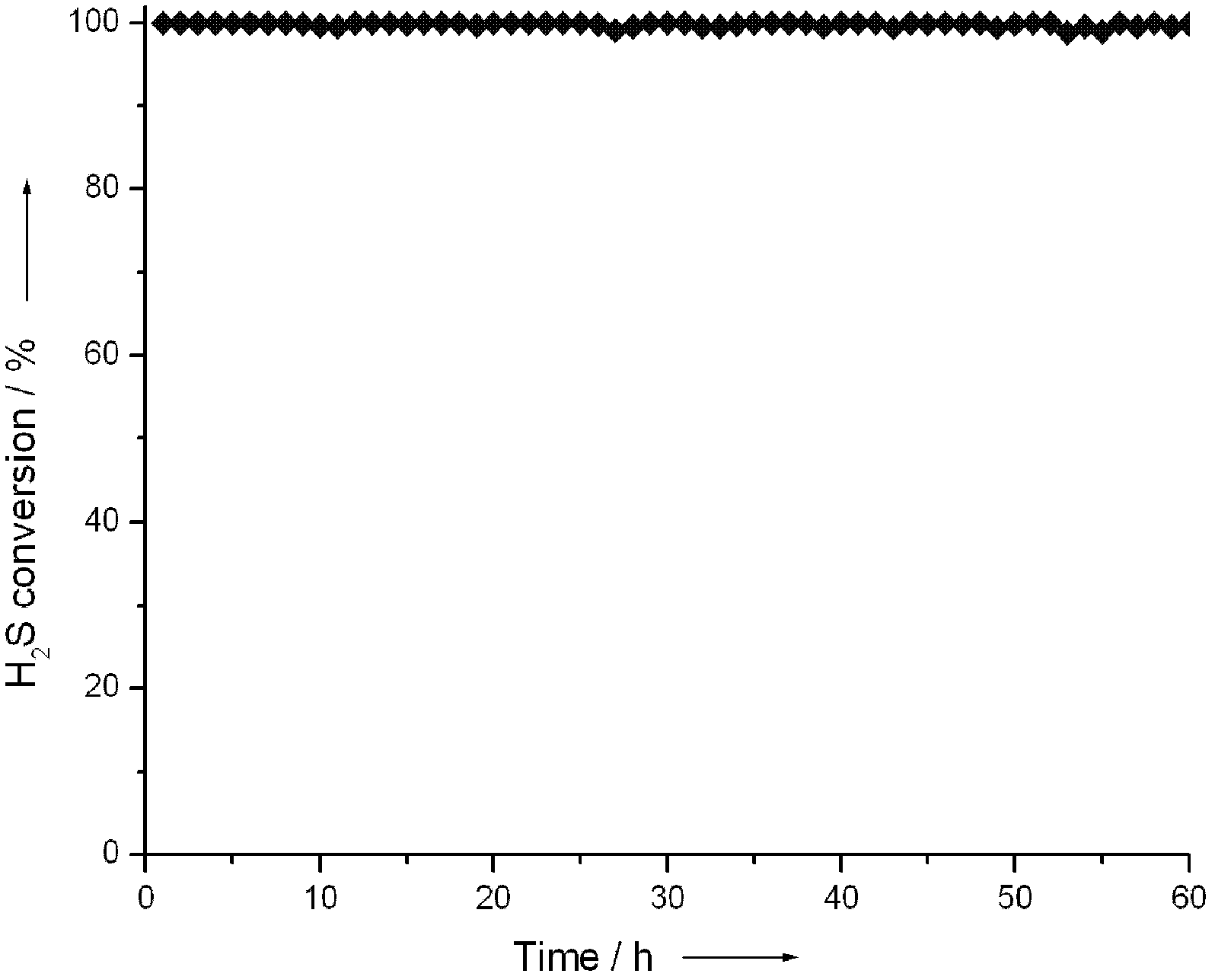
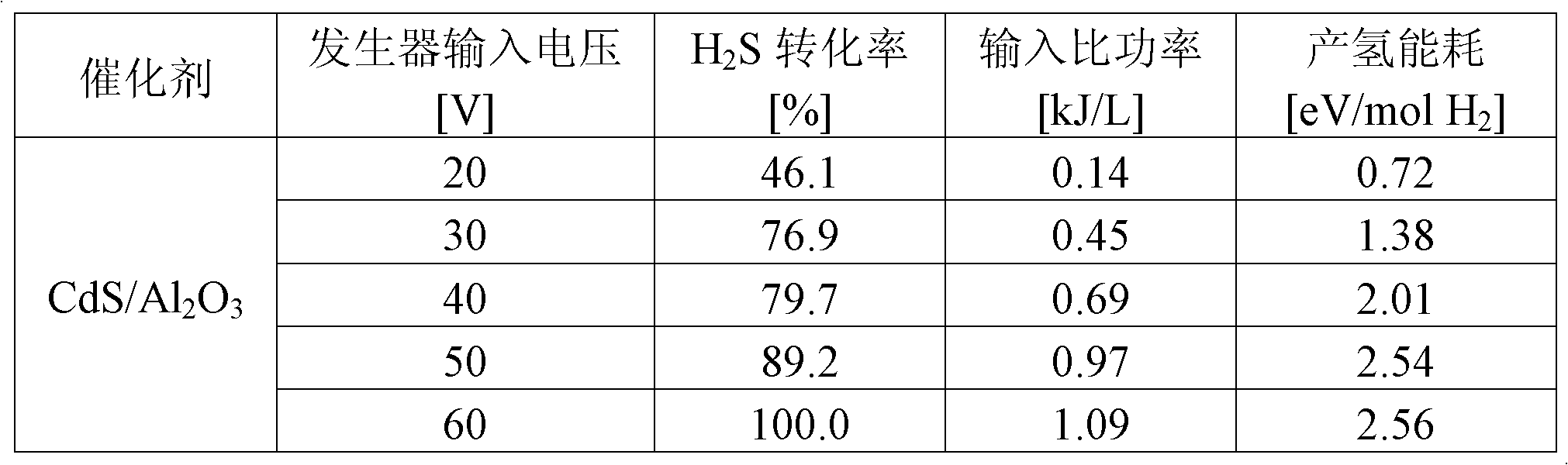
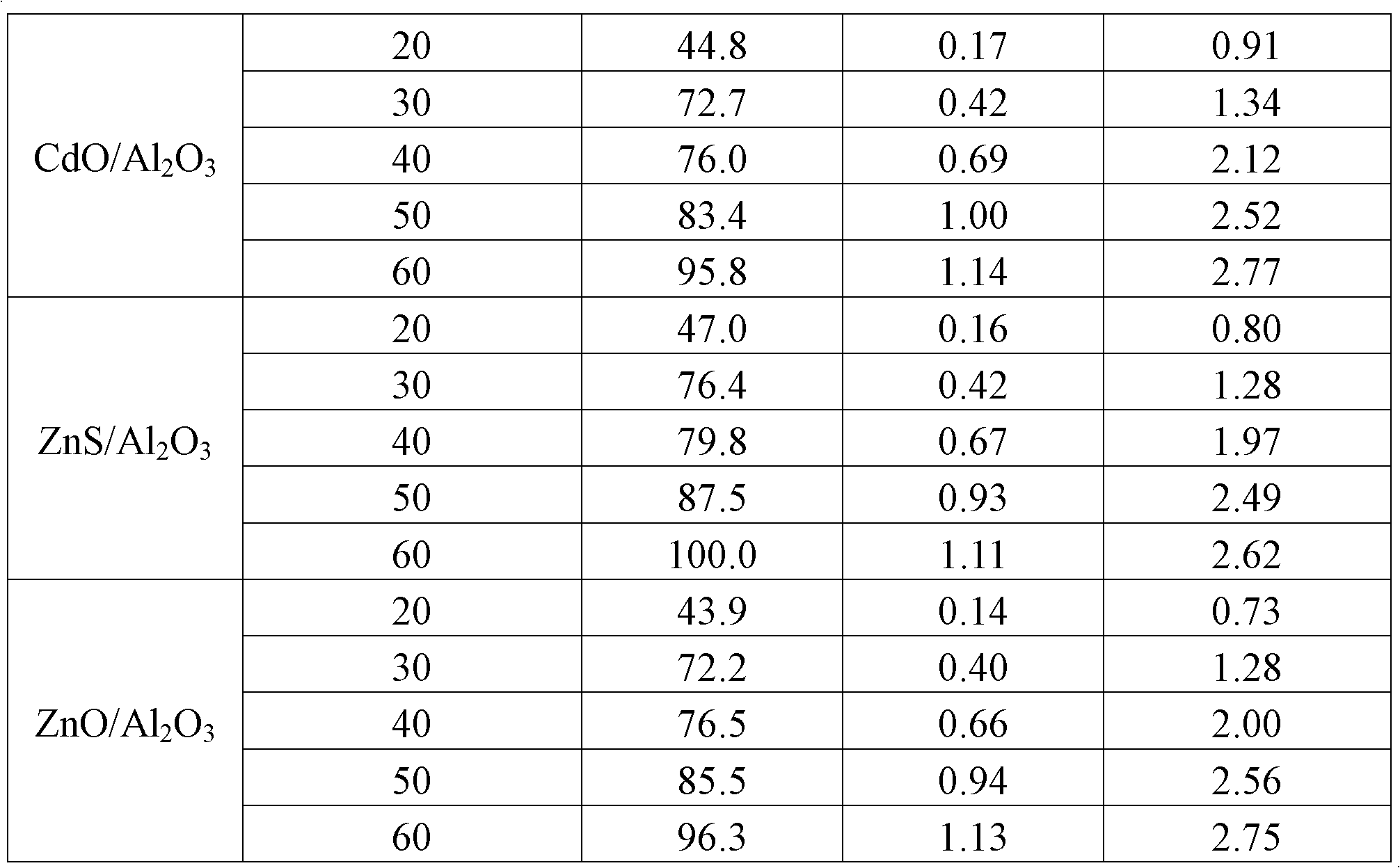
Image
Examples
Embodiment 1
[0016] Catalyst preparation: commercially available titanium dioxide, silicon dioxide and aluminum oxide solid powders are molded under pressure, and then sieved to obtain 20-40 mesh particles.
Embodiment 2
[0018] Weigh 1.50 grams of γ-Al with a particle size of 20 to 40 mesh 2 o 3 Carrier (specific surface area 270m 2 / g), take 0.40 g of Cd(NO 3 ) 2 4H 2 O was dissolved in 1.5 ml of deionized water, and the solution was slowly dropped into the carrier and stirred evenly, impregnated at room temperature for 8 hours, and then dried in an oven at 120°C for 12 hours, and the obtained solid was dried in a muffle furnace at 450°C in an air atmosphere After 5 hours of calcination, it was lowered to room temperature, and the obtained catalyst was marked as CdO / Al 2 o 3 . The same method can be used to prepare ZnO / Al 2 o 3 .
Embodiment 3
[0020] With the CdO / Al obtained in embodiment 3 2 o 3 Put it into the quartz tube for vulcanization and pass through the vulcanizing agent (10%H 2 S / Ar), rising to 400°C in 20 minutes and holding for 100 minutes. Obtain the catalyst containing 10% (mass fraction) cadmium sulfide supported by alumina, denoted as CdS / Al 2 o 3 . ZnS / Al 2 o 3 Prepared by the same method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com