Acylhydrazone zinc protoporphyrin, and synthesis and application of complex thereof
A technology of porphyrin complexes and porphyrin compounds, applied in porphine/acridine porphine, chemical instruments and methods, electrolytic capacitors, etc., can solve the problems of low conversion efficiency of porphyrin photosensitive dyes, and achieve good photoelectric performance and environmental protection Low pollution, simple and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
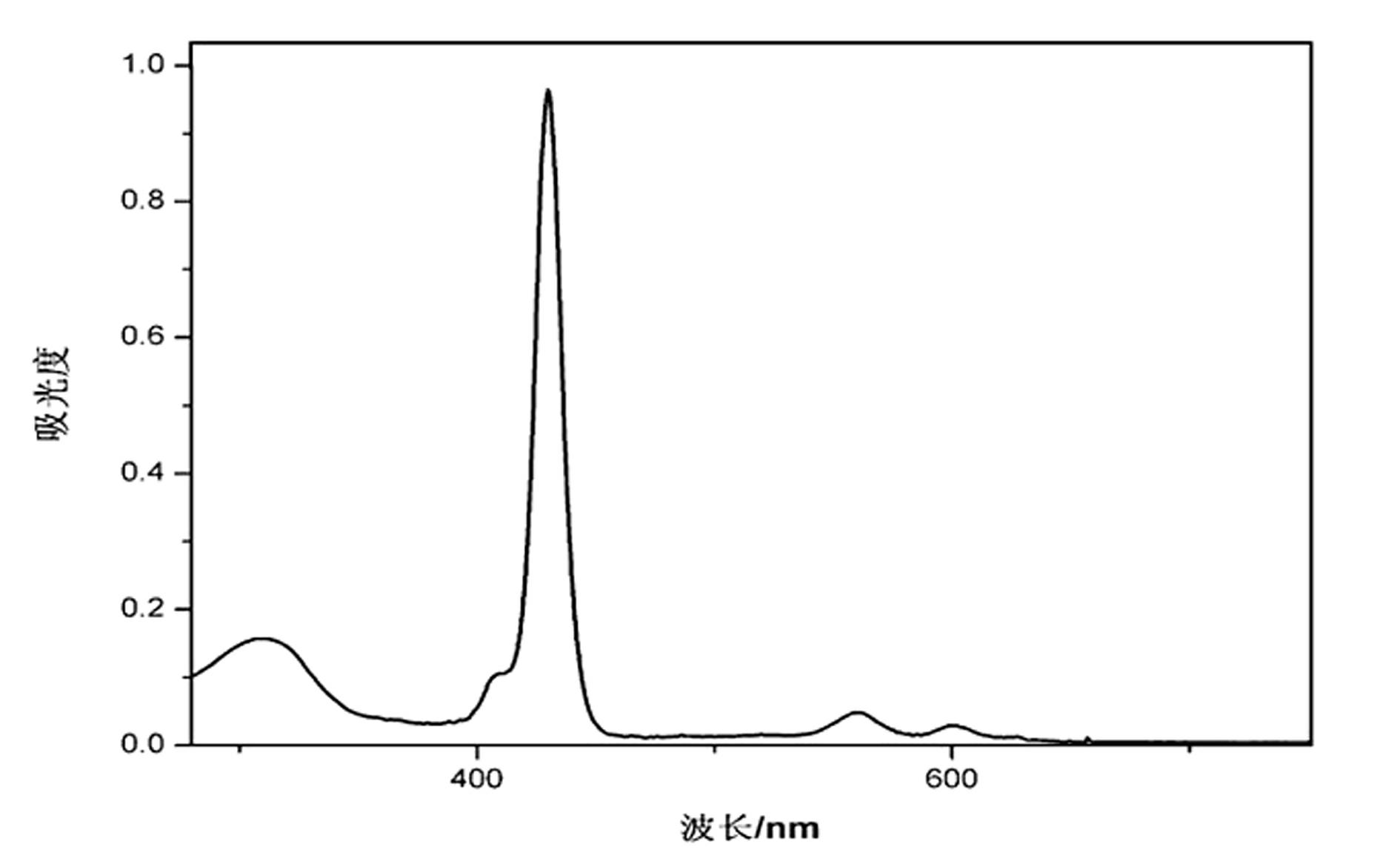
[0059] Embodiment 1, dyestuff I——5,10,15,20-tetra[(4-(2-pyridinecarbaldehyde-benzoylhydrazone)] base zinc porphyrin synthesis
[0060] (1) Freshly distilled pyrrole (15mmol, 1.04mL) and methyl 4-formylbenzoate (15mmol, 2.4624g) were stirred and dissolved in 1500mL of dichloromethane, and methanesulfonic acid (10.4mmol, 0.68mL) was added dropwise, React for 30 minutes, add dichlorodicyano-p-benzoquinone (13.2mmol, 3g), stir for 1 minute, then add 3mL triethylamine to neutralize methanesulfonic acid (MSA), make the pH of the system = 7.0, and the reaction is completed. Concentrate the reaction solution by rotary evaporation, install it on the upper end of a silica gel column (diameter 5cm×length 15cm), elute with dichloromethane containing 1% methanol until the solution is light brown, collect all eluted components, and concentrate by rotary evaporation; Put the concentrated solution on the upper end of a basic alumina column (diameter 5cm x length 12cm), elute with dichlorometh...
Embodiment 2
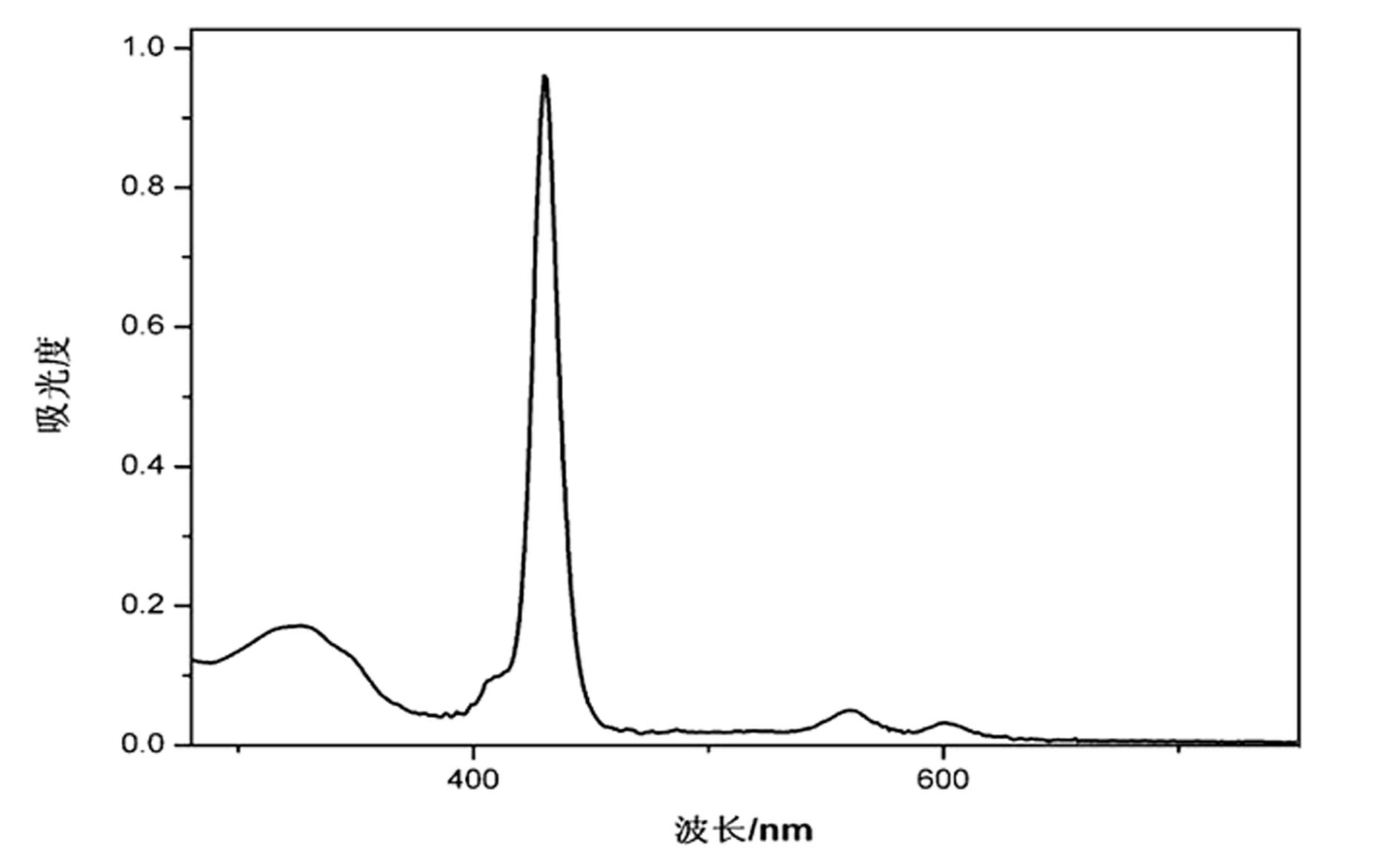
[0067] Embodiment 2, dye II——5,10,15,20-tetra[(4-(2-acetylbenzimidazole-benzoylhydrazone)] base zinc porphyrin synthesis
[0068] Steps (1), (2), and (3) are the same as in Example 1.
[0069] (4) Stir and dissolve 5,10,15,20-tetrakis(4-hydrazino)phenyl zinc porphyrin (0.4mmol, 0.3640g) in 35mL DMF; dissolve 2-acetylbenzimidazole (3.2mmol, 0.5128g) was dissolved in 45mL of ethanol at 60~70°C, then 1.5ml of glacial acetic acid (catalyst) was added dropwise, the two solutions were mixed under stirring, and the mixture was refluxed at 105°C. Followed by TLC, the reaction took 16 hours. After the reaction is completed, the solvent is removed, an appropriate amount of ether is added, and a large amount of purple-red precipitate is precipitated, filtered with suction, recrystallized with DMF-water (volume ratio 1:1), the green precipitate is collected, and dried at a constant temperature of 100°C to obtain the product dark purple powder II ——5,10,15,20-Tetrakis[(4-(2-acetylbenzimi...
Embodiment 3
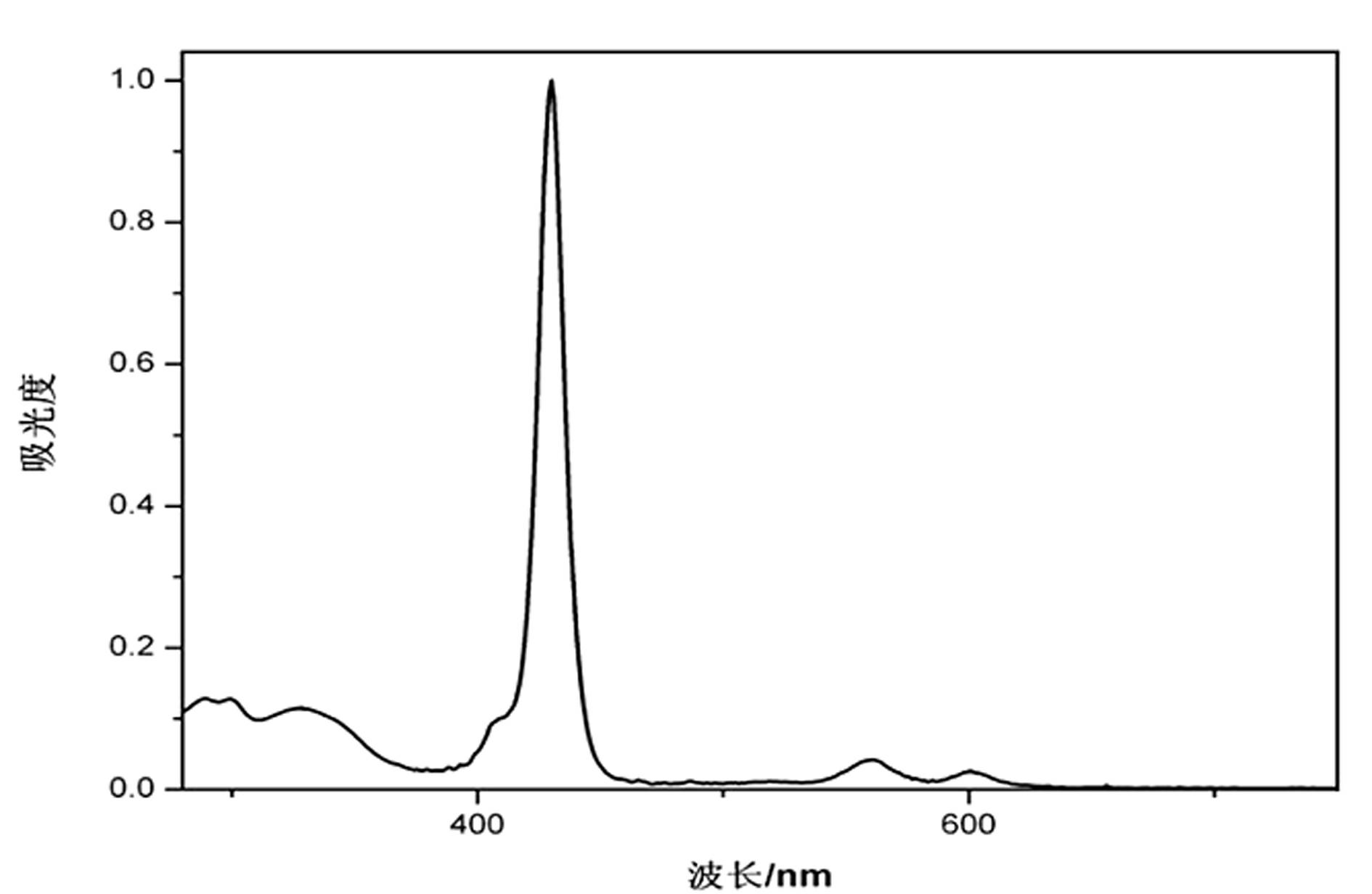
[0074] Embodiment 3, dye III——5,10,15,20-tetra[(4-(salicylaldehyde-benzoylhydrazone)] base zinc porphyrin synthesis
[0075] Steps (1), (2), and (3) are the same as in Example 1.
[0076] (4) Stir and dissolve 5,10,15,20-tetrakis(4-hydrazino)phenyl zinc porphyrin (0.5mmol, 0.4550g) in 40mL DMF; dissolve salicylaldehyde (4mmol, 0.3908g) In 35 mL of ethanol, the two solutions were mixed under stirring, and the mixture was refluxed at 100°C. Followed by TLC, the reaction took 6 hours. After the reaction is complete, remove the solvent, add an appropriate amount of ether, let it stand still, and precipitate a large amount of purple precipitate, filter it with suction, recrystallize with acetone-ethanol (volume ratio 1:1), and collect the precipitated purple powder III——5,10,15,20- Tetra[(4-(salicylaldehyde-benzoylhydrazone)]-based zinc porphyrin. Yield 88%.
[0077] Its structure is as follows:
[0078]
[0079] The product is detected by a Varian nuclear magnetic resonance...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com