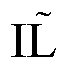Application of H22 liver cancer cell autophagosome to preparation of liver cancer therapeutic vaccine
A therapeutic vaccine and liver cancer cell technology, applied in the field of liver cancer vaccine development to achieve the effect of inhibiting the growth of tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of autophagosome DRibbles in H22 / BNL liver cancer cells.
[0040] 1. Culture of H22 / BNL liver cancer cells:
[0041]Take the H22 / BNL cells frozen in liquid nitrogen, add 1640 culture medium containing 10% (V / V) fetal bovine serum, 100U / ml penicillin and 100U / ml streptomycin immediately after thawing in a water bath at 40°C, and resuspend the cells to 1×10 6 individual / ml, and transfer it into a 500ml cell culture flask, at 37°C, 5% (V / V) CO 2 Cultured in a constant temperature incubator, the medium was changed every 1 to 2 days, and routinely digested and passaged with 0.25% trypsin;
[0042] 2. Treatment of H22 / BNL liver cancer cells:
[0043] After the cells cultured in step (1) adhere well to the wall and have a moderate density, add 100 nmol / L of rapamycin, 200 nmol / L of Velcade, and ammonium chloride (NH 4 CL) 30mmol / L combined treatment for 16 hours, induced autophagosomes - DRibbles. And set up a control group (only adding culture medi...
Embodiment 2
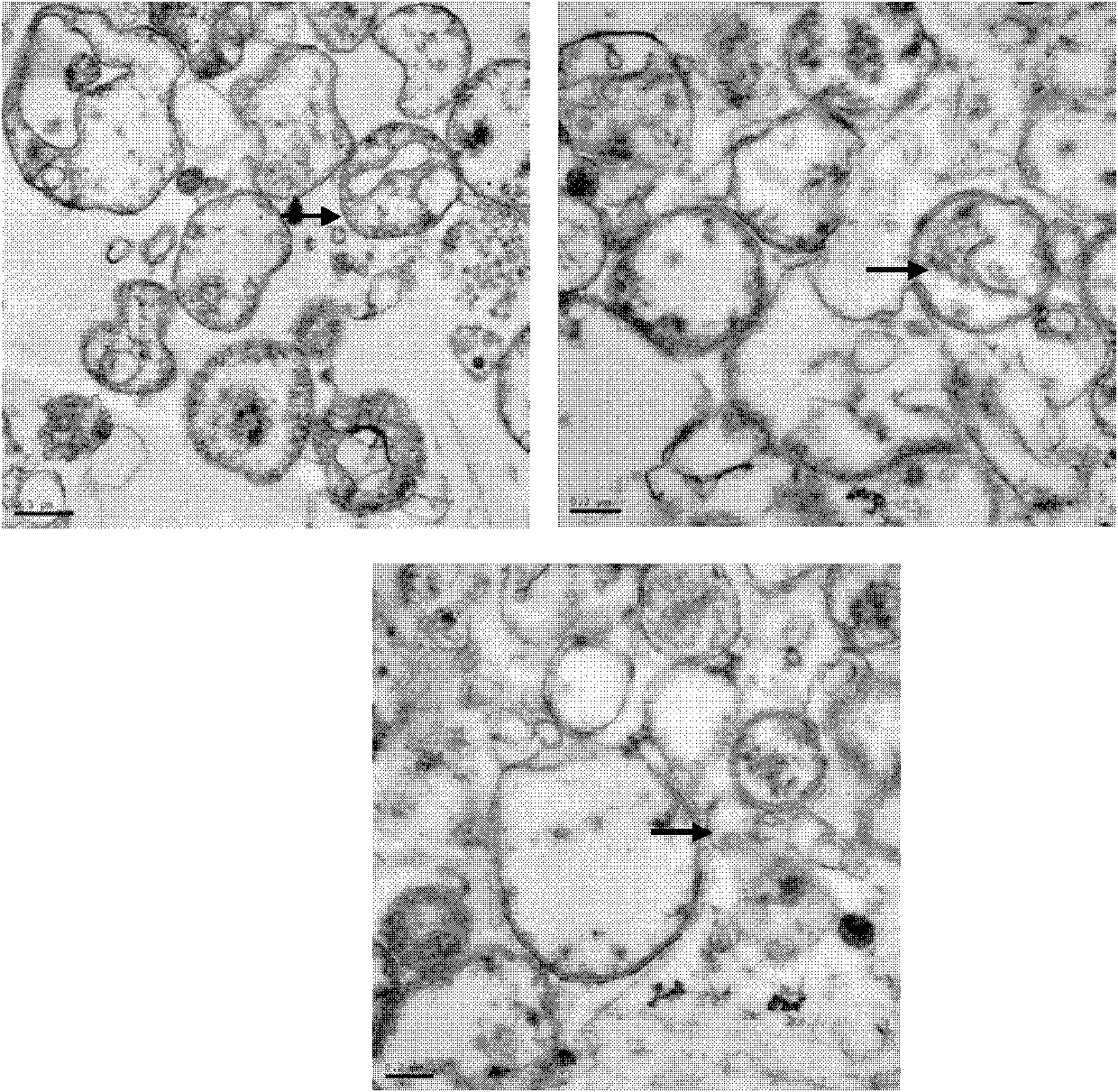
[0052] Example 2: Transmission Electron Microscopy Identification of Morphology of DRibbles.
[0053] The extracted DRibbles were centrifuged at high speed to compress them tightly, the supernatant was discarded, the precipitate was fixed with 2.5% (V / V) glutaraldehyde, and sent to the electron microscope room for processing, and the morphology of DRibbles was observed on the microscope. Such as figure 1 Shown: Under the electron microscope, small bodies with a membrane structure can be seen, with an average diameter of about 200-300 nm (pointed by the arrow), which proves that the autophagosomes of H22 liver cancer cells are effectively recruited.
Embodiment 3
[0054] Example 3: Determination of total protein content in DRibbles (Bradford method).
[0055] 1) Completely dissolve the protein standard, take 25 μl and dilute to 100 μl with PBS;
[0056] 2) Add 0, 1, 2, 4, 8, 12, 16, and 20 μl of the standard into the 96-well plate, and add PBS to make up to 20 μl;
[0057] 3) Freeze and thaw the DRibble to be tested repeatedly in advance, centrifuge to take the supernatant, add an appropriate volume of sample to a 96-well plate, and add PBS to make up to 20 μl;
[0058] 4) Add 200 μL of LG250 staining solution to each well, and place at room temperature for 3-5 minutes;
[0059] 5) Measure A595 with a microplate reader, or the absorbance of other wavelengths between 560-610nm;
[0060] 6) Use the software ElisaCalc to draw a standard curve, and calculate the protein concentration in the sample according to the standard curve, and the result is about 1-3 μg / μl.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com