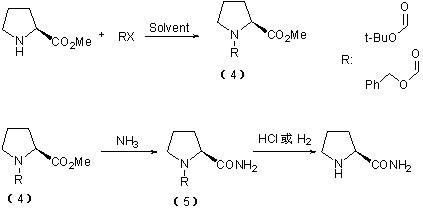
Method for preparing L-prolinamide and intermediate thereof
A technology for prolineamide and proline is applied in the field of preparing chiral drug intermediate L-prolineamide and its intermediates, which can solve the problems of pollution, long period of L-prolineamide and the like, achieve complete reaction conversion, The effect of few crystallization steps and short reaction period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of L-proline-N-carboxy-cyclic anhydride (NCA)
[0032]
[0033] (3)
[0034] In a dry 500ml three-neck flask, add 250ml dry THF under nitrogen flow, add L-proline (15g, 0.13mol) and triphosgene (13.5g, 0.046mol) at 20~25°C to obtain a suspension. Slowly raise the temperature of the suspension to 30~40°C while stirring o C, and continue to maintain for 70 minutes. Until the reaction solution becomes a transparent solution, and then stirred at constant temperature for 30 minutes, L-proline carbamoyl chloride is formed. Then concentrate under reduced pressure (10~20 o °C) for 30 minutes to remove the HCl gas. Cool the reaction solution to 0 o C was added dropwise dry triethylamine (15.5g, 0.15mol), and the addition was completed in 30 minutes. The reactant is between 0~5 o C continued to stir for 30 minutes. It was filtered under nitrogen, and the solid (triethylamine hydrochloride) was washed with 50 ml of dry THF. That is...
Embodiment 2
[0035] Example 2: Preparation of L-proline-N-carboxy-anhydride (NCA)
[0036] In a dry 1000ml three-necked flask, add L-proline (30g, 0.26mol) and 300ml dry methyl tert-butyl ether to obtain a suspension. Under nitrogen flow, add THF solution of triphosgene dropwise at 20-25°C (triphosgene 30g, 0.10mol; THF150ml) and control the temperature of the suspension at 30-35°C o C was stirred for 2 hours. The reaction solution becomes a transparent solution, and then it is incubated and stirred for 30 minutes, and L-proline carbamoyl chloride is formed. Then it was concentrated under reduced pressure to remove the solvent and the generated HCl. Add 250ml of toluene, keep 5~10 o C, diethylbenzylamine (58.7 g, 0.36 mol) was added dropwise, and the addition was completed in 40 minutes. The reaction was stirred for an additional 30 minutes at this temperature. Filter under nitrogen protection, and wash the solid (diethylbenzylamine hydrochloride) with 150ml of toluene. The toluene s...
Embodiment 3
[0037] Example 3: Preparation of L-proline-N-carboxy-cyclic anhydride (NCA)
[0038] In a dry 500ml three-necked flask, add L-proline (15g, 0.13mol) and 150ml dry chloroform to obtain a suspension, under nitrogen flow, control the temperature at 10~15°C, and add 2M phosgene toluene solution dropwise (78ml, 0.156mol) and then stirred at 20-25°C for 3 hours to obtain a transparent solution, then kept stirring for 30 minutes to obtain L-proline carbamoyl chloride solution, and then concentrated to dryness under reduced pressure. Add 150ml methyl tert-butyl ether and keep it at 0~5 o C was added dropwise to dry pyridine (20.54 g, 0.26 mol), and the addition was complete in 25 minutes. Reactants at 5 o C continued to stir for 30 minutes. It was filtered under nitrogen protection, and the solid (pyridine hydrochloride) was washed with 50 ml of methyl tert-butyl ether. The obtained L-proline-N-carboxy-anhydride (NCA) in methyl tert-butyl ether solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com