Stable HRP enzymatic enhanced chemiluminescent substrate solution
A technology of enzymatic chemiluminescence and substrate solution, which is applied in the field of immunoassay, can solve the problems of limiting the application of chemiluminescence immunoassay, low luminous intensity, poor stability, etc., and achieves short reaction time, high luminous intensity value and high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 Chemiluminescence substrate solution preparation
[0030] Preparation of buffer: 0.1mol / L Tris-HCl buffer, pH8.4. 0.01mol / L PBS buffer, pH7.4, the specific formula is as follows:
[0031] Liquid A (luminescent agent solution):
[0032]
[0033] B liquid (oxidant solution):
[0034]
[0035]
[0036] The following method can be used to prepare 1000mL of liquid A (luminescent agent solution) of chemiluminescence substrate: first, dissolve 0.2417g of 4-(1,2,4-triazol-1-yl)phenol in 10mL of N,N-di In methylformamide, mix well; take 0.1991g of luminol monosodium salt, 0.1100g of p-iodophenol, 1.8610g of edetate disodium salt, 0.0144g of sodium benzoate, and 0.1755g of sodium chloride; -(1,2,4-Triazol-1-yl)phenol solution and various substances were added to Tris-HCl buffer solution, and the volume was adjusted to 1000ml with Tris-HCl buffer solution, mixed well, and stored at 4°C.
[0037] The preparation of 1000 mL of liquid B (oxidizing agent solut...
Embodiment 2
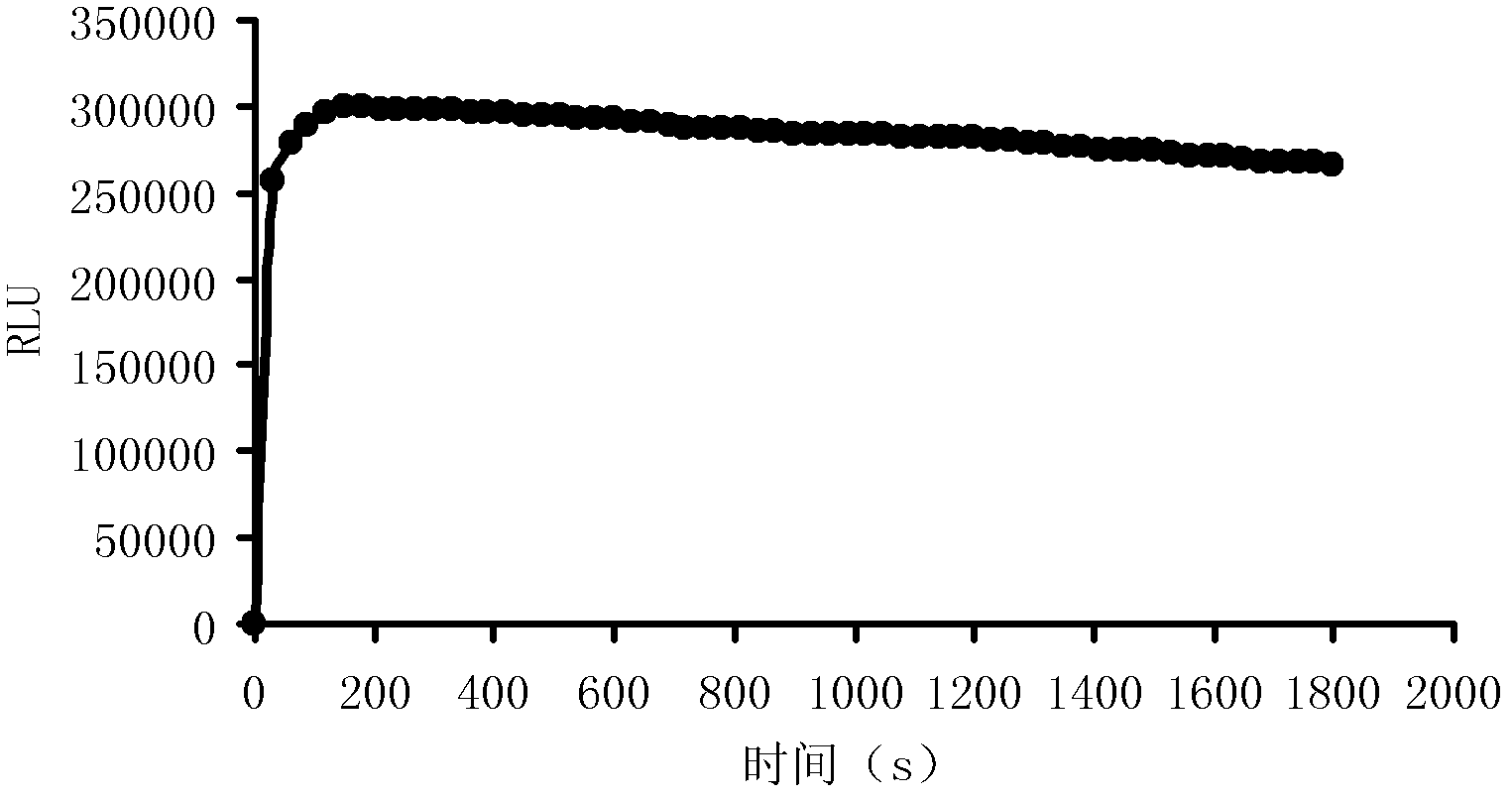
[0038] Example 2 Enzyme-catalyzed chemiluminescent reaction kinetics curve
[0039] Add 10 μL of HRP (10 ng / mL) to the microwell plate, add 50 μL of the luminescent substrate A solution and 50 μL of the luminescent substrate B solution prepared in Example 1 to each well, and quickly detect the luminous intensity (RLU), and continuously detect for 30 minutes, draw the reaction kinetics curve, the results are shown in the attached figure 1 ,from figure 1 It can be seen that the luminescent substrate solution prepared in Example 1, the luminous intensity of the reaction reaches the maximum in about 3 minutes, and then there is a long plateau period. After 30 minutes of detection, it still maintains a high luminous intensity. This shows that the luminescent substrate solution of the present invention has high reaction stability, and can effectively reduce errors when applied to a detection system.
Embodiment 3
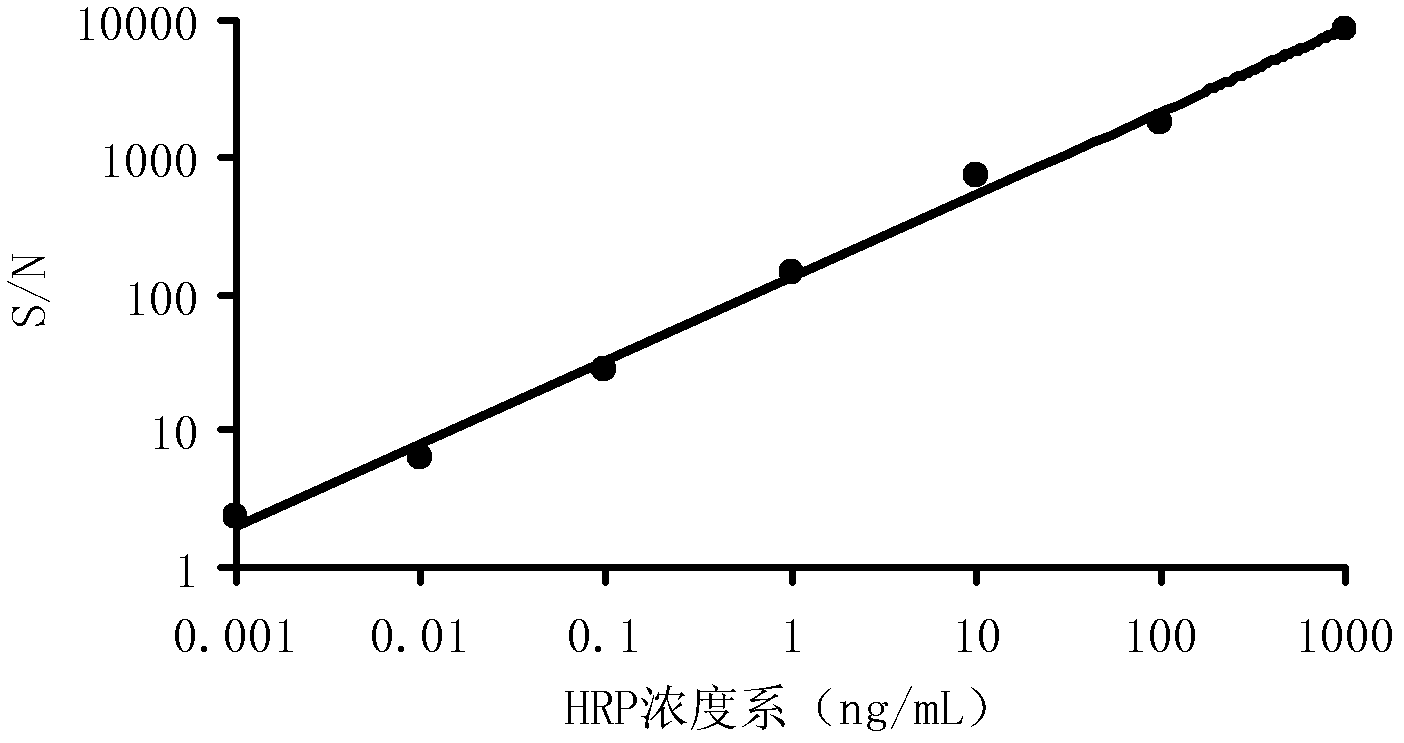
[0040] Embodiment 3 Sensitivity research
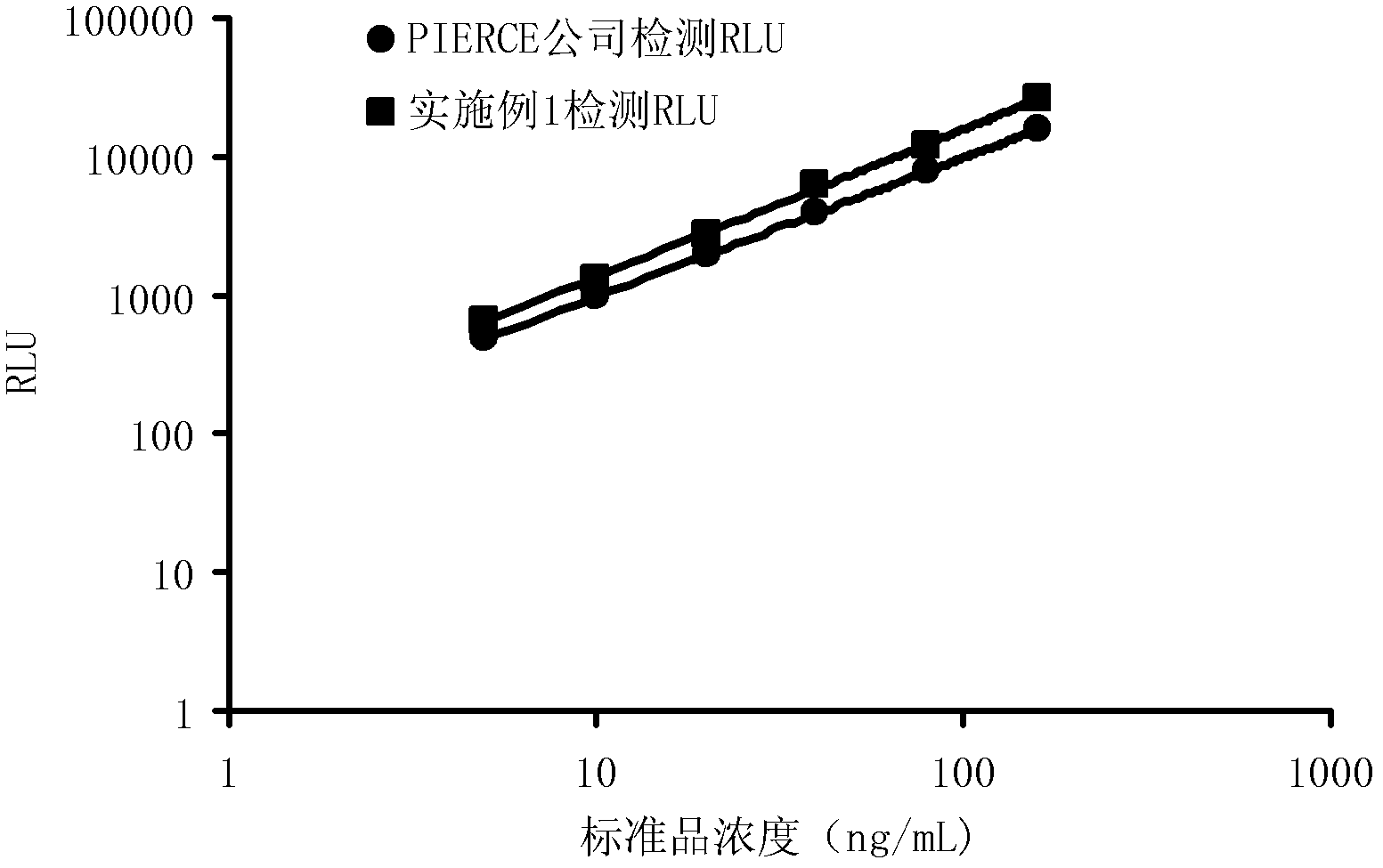
[0041] Dilute the newly prepared 1mg / mL HRP solution with PBS buffer, and select the concentration from 100ng / mL to 0.001ng / mL for detection. detection concentration), and then add 50 μL of luminescent substrate A solution and 50 μL of luminescent substrate B solution prepared in Example 1 to each well, and detect RLU after standing for 5 minutes. The results of three detections in parallel are represented by S / N, as shown in Table 1 and attached figure 2 shown. From Table 1 and figure 2 It can be seen that the concentration of HRP is positively correlated with the signal-to-noise ratio. With the decrease of the HRP concentration, the signal-to-noise ratio of the luminous intensity is also decreasing. The logarithm of S / N is the ordinate, and the concentration of HRP is the abscissa In the drawing, each corresponding point is almost on a straight line, which shows that the luminescent substrate prepared in Example 1 has a strong ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com