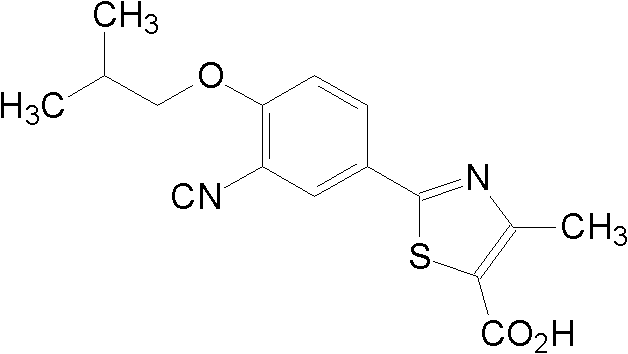
Medicinal composition containing febuxostat crystal and preparation method thereof
A technology of febuxostat and its composition, which is applied in the field of drug synthesis, can solve problems such as surfactant toxicity, unprepared stability investigation, and complicated preparation process, so as to reduce the loss of raw materials, ensure the dissolution rate of drugs, and simplify the process. The effect of the operating process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
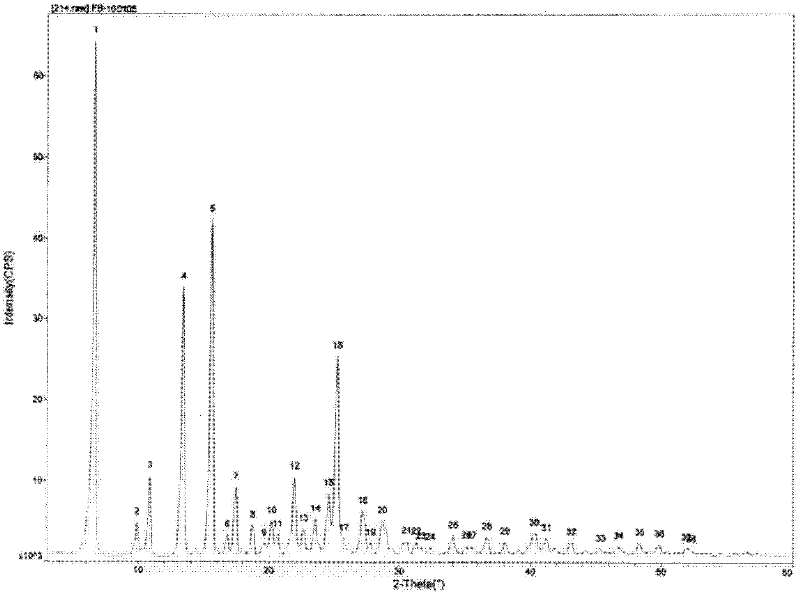
[0038] Preparation of febuxostat in the above crystal form: add 120mL of 2-butanone and 20g of crude febuxostat to a 250mL round bottom flask, start magnetic stirring, heat the water bath to reflux, continue stirring for 30 minutes, stop heating, and continue stirring until Crystallize at room temperature for 3 hours, filter with suction, and vacuum-dry the filter cake at 55° C. to constant weight, with a yield of 91.4%.
[0039]
[0040] Preparation:
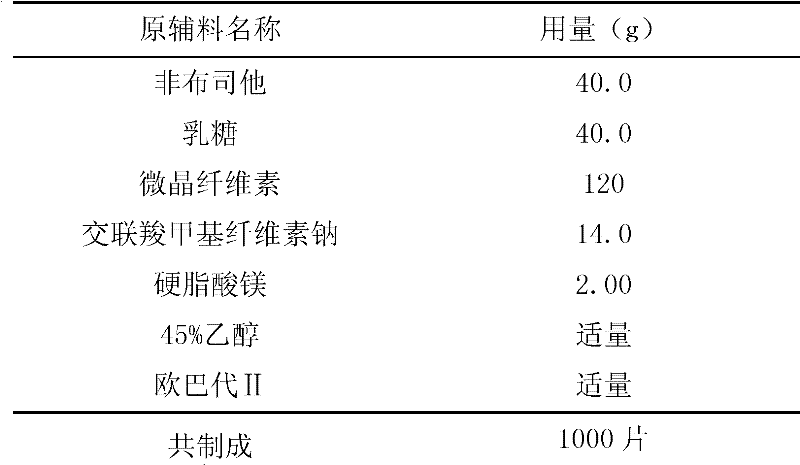
[0041] 1. Grind the febuxostat raw material to an average particle size of 100 μm and set aside.
[0042] 2. Separately pass lactose, microcrystalline cellulose, croscarmellose sodium cellulose, and magnesium stearate through 80 mesh for later use.
[0043] 3. Configure the mass percentage as 45% ethanol solution for later use.
[0044] 4. Mix febuxostat, lactose, microcrystalline cellulose, and croscarmellose sodium in the bottom material of the fluidized bed granulator in a boiling state, spray an appropriate amount of 4...
Embodiment 2
[0048] Preparation of febuxostat in the above crystal form: add 120mL of 2-butanone and 20g of crude febuxostat to a 250mL round bottom flask, start magnetic stirring, heat the water bath to reflux, continue stirring for 30 minutes, stop heating, and continue stirring until Crystallize at room temperature for 4 hours, filter with suction, and vacuum-dry the filter cake at 55° C. to constant weight, with a yield of 92.9%.
[0049]
[0050] Preparation:
[0051] 1. Grind the febuxostat raw material to an average particle size of 85 μm and set aside.
[0052] 2. Separately pass lactose, microcrystalline cellulose, croscarmellose sodium cellulose, and magnesium stearate through 80 mesh for later use.
[0053] 3. Configure the mass percentage as 10% ethanol solution for later use.
[0054] 4. Mix febuxostat, lactose, microcrystalline cellulose, and croscarmellose sodium in the bottom material of the fluidized bed granulator in a boiling state, spray an appropriate amount of 45...
Embodiment 3
[0058] Preparation of febuxostat in the above crystal form: Add 120mL of methyl tert-butyl ketone and 20g of crude febuxostat into a 250mL round bottom flask, start magnetic stirring, heat the oil bath to reflux, continue stirring for 60 minutes, and then stop heating. Continue stirring to room temperature, crystallize for 5 hours, filter with suction, and vacuum-dry the filter cake at 60° C. to constant weight, with a yield of 89.8%.
[0059]
[0060]
[0061] Preparation:
[0062] 1. Grind the febuxostat raw material to an average particle size of 75 μm and set aside.
[0063] 2. Separately pass lactose, microcrystalline cellulose, croscarmellose sodium cellulose, and magnesium stearate through 80 mesh for later use.
[0064] 3. Configure the mass percentage as 60% ethanol solution for later use.
[0065] 4. Mix febuxostat, lactose, microcrystalline cellulose, and croscarmellose sodium in the bottom material of the fluidized bed granulator in a boiling state, spray a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com