A kind of esomeprazole magnesium intestinal capsule and preparation method thereof
A technology of esomeprazole magnesium enteric coating and esomeprazole magnesium, which is applied in the field of esomeprazole magnesium enteric coating capsule and its preparation, can solve the problems of drug oxidative degradation, etc., to prevent oxidative degradation, facilitate Marketing and clinical use, the effect of preventing decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 The preparation of esomeprazole magnesium enteric-coated pellets
[0024] (1) Preparation of blank pellet cores
[0025] Starch 400g
[0026] Sucrose 477g
[0028] Syrup (60%) 200g
[0029] It is prepared with production equipment such as centrifugal granulator, extrusion extrusion spheronizer, high-efficiency coating machine and fluidized bed coating granulator. The prepared blank pellet cores are screened, and pellets with a particle diameter of 0.4-0.6 mm are selected for drug packaging.
[0030] (2) Preparation of the drug-loaded layer
[0031] Esomeprazole Magnesium 1000g
[0032] Microcrystalline Cellulose 300g
[0034] Polysorbate 80 10g
[0035] Hydroxypropyl Methyl Cellulose 150g
[0036] Water 10000g
[0037]Pass esomeprazole magnesium through a 200-mesh sieve for later use, dissolve sodium hydroxide in about 2kg of water, disperse the hypromellose with hot water, add the remainin...
example 1
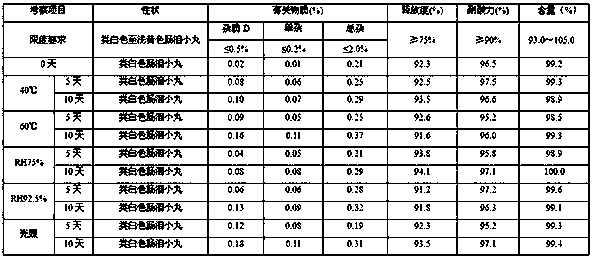
[0083]Experiments were carried out on the influencing factors of high temperature (temperature 60°C±2°C), high humidity (RH75%, RH92.5%) and light (illuminance 4500±500LX).
[0084] Influencing factor test results
[0085]
example 2
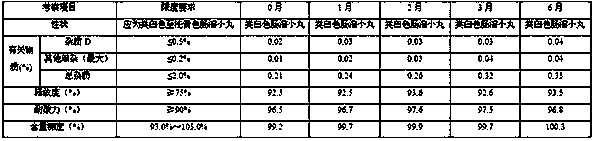
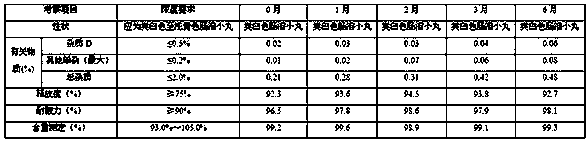
[0087] Take the example sample and carry out acceleration (at a temperature of 40°C±2°C and a relative humidity of 75%±5%) for 6 months and a long-term period (at a temperature of 25°C±2°C and a relative humidity of 60%±5%) according to the package on the market. 10% condition) 6-month pilot study.
[0088] Accelerated test (temperature 40℃±2℃, relative humidity 75%±5%) 6-month test results
[0089]
[0090] Long-term (temperature 25 ℃ ± 2 ℃, relative humidity 60% ± 10%) test results for 6 months
[0091]
[0092] According to the experimental data, according to the results of the test and investigation of the influencing factors of this product, the appearance of the sample has not changed, and the measured indicators have no obvious changes. It has been placed under high temperature, high humidity, and light conditions for 10 days, except for a slight increase in related substances under light conditions. There was no significant change in other indicators. Accelerat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com