C8 aromatic hydrocarbon isomerization catalyst, preparation method and application thereof
A technology of aromatics isomerization and catalyst, which is applied in the field of conversion of ethylbenzene in C8 aromatics, can solve the problems of product yield reduction and xylene loss, and achieve the effects of increasing yield, reducing energy consumption and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] Provide a kind of specific preparation method of catalyst of the present invention below, but not limited to this method, concrete steps are:
[0022] (1) loading the rare earth on the molecular sieve, drying and roasting to obtain the rare earth modified molecular sieve;
[0023] (2) loading the halogen on the inorganic refractory oxide, drying and roasting to obtain the halogen modified inorganic refractory oxide;
[0024] (3) Fully kneading rare earth modified molecular sieves, halogen modified inorganic refractory oxides, extrusion aids, water and peptizing agent into a plastic paste, extrusion molding, drying and roasting to obtain the catalyst carrier of the present invention.
[0025] (4) The active metal-containing component is loaded on the carrier by a conventional impregnation method, and then dried and calcined to obtain the xylene isomerization catalyst of the present invention.
[0026] The rare earth described in step (1) can be loaded on the molecular s...
Embodiment 1
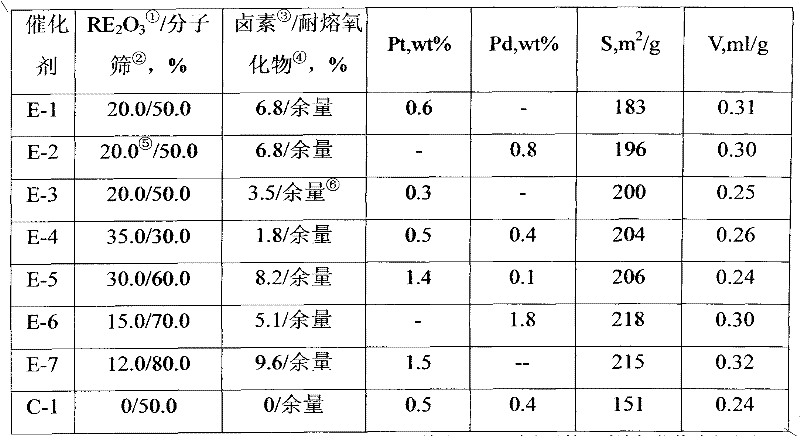
[0036] The preparation of catalyst E-1 of the present invention
[0037] (1) Preparation of EUO molecular sieves.
[0038] The EUO molecular sieve used in the present invention is prepared according to the method of US Pat. 2 / g, pore volume 0.19mL / g.
[0039] The zeolite synthesized above contains alkali metal or alkaline earth metal cations, which are exchanged with ammonium cations, and then roasted in the air at 316°C to 540°C for 1 to 10 hours, and the obtained acidic molecular sieve is numbered S-1, which forms acidic zeolite are well known in the art.
[0040] (2) getting 1000 grams of mass concentration as lanthanum nitrate solution of 20% (calculated as lanthanum oxide) and 800 grams of S-1 molecular sieves is fully mixed, then dried at 130°C for 24 hours, and roasted at 750°C for 3 hours, A modified S-1 molecular sieve with a mass content of lanthanum oxide of 20% was obtained. The number is LS-1 molecular sieve.
Embodiment 2
[0045] The preparation of catalyst E-2 of the present invention
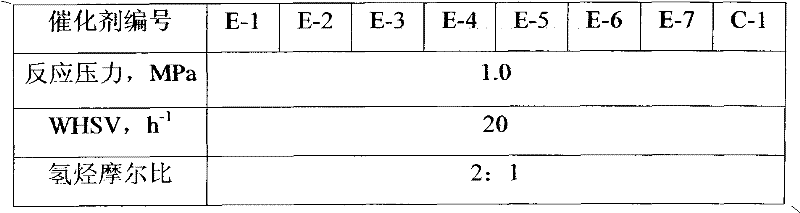
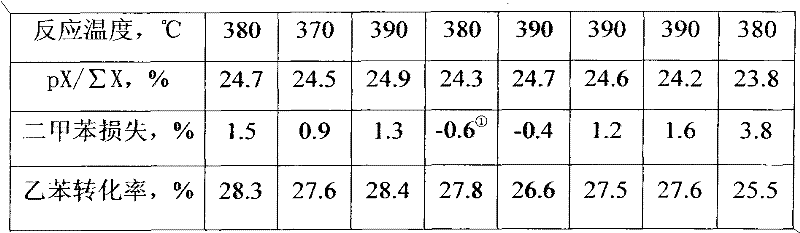
[0046] The preparation process of the catalyst E-2 of the present invention is the same as in Example 1, except that cerium nitrate is used to replace lanthanum nitrate, and the prepared catalyst of the present invention is numbered E-2, its physicochemical properties are shown in Table 2, and the reaction results are shown in Table 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com