Pharmaceutical preparations comprising nitrocatechol derivatives and methods for their preparation
The technology of a pharmaceutical preparation and nitrophenyl is applied in the field of pharmaceutical preparations including nitrocatechol derivatives and the preparation thereof, and can solve the problems of poor flow characteristics, low bulk density, increased difficulty and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
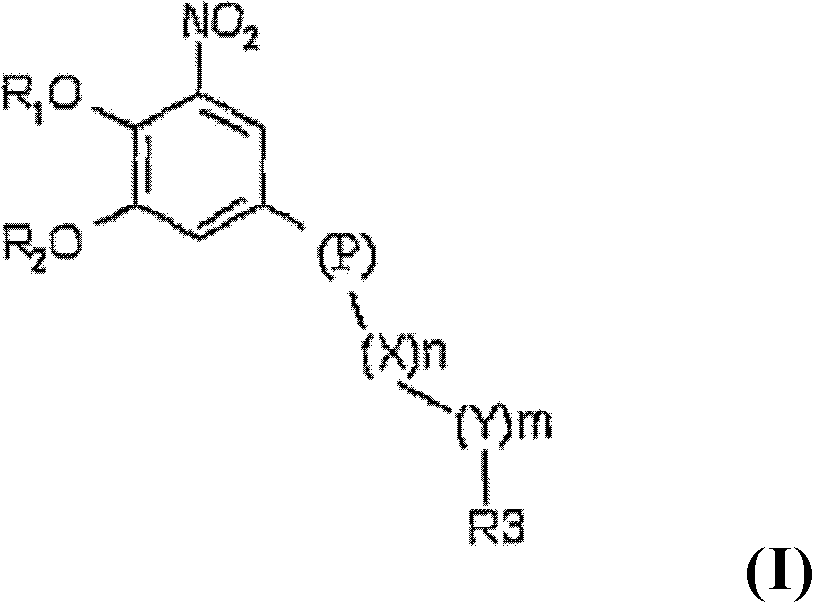
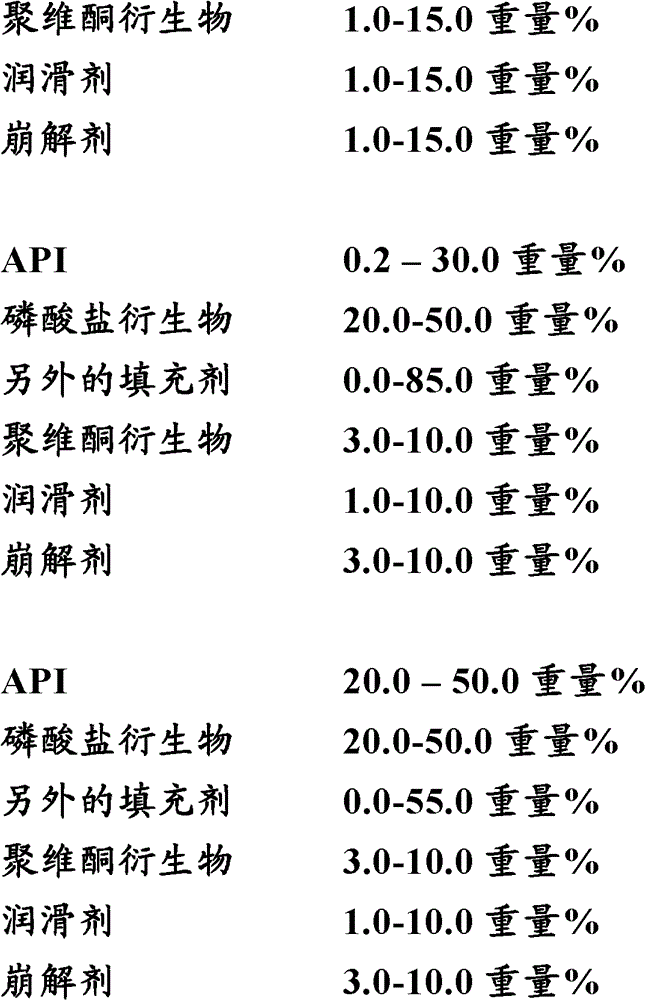
[0078]Four laboratory-scale high-dose capsules were prepared by first mixing the API with dicalcium phosphate and / or microcrystalline cellulose, croscarmellose sodium and / or, povidone, and / or pregelatinized starch in The amounts shown in Table 1 below were mixed in a laboratory scale high shear mixer (Stephan). The API used in these examples is 2,5-dichloro-3-(5-(3,4-dihydroxy-5-nitrophenyl)-1,2,4-oxadiazol-3-yl )-4,6-lutidine 1-oxide. Purified water was added to each mixture, and the mixture was granulated.
[0079] The granules were then dried in a laboratory scale fluid bed dryer (Aeromat). The dried granules were sieved and mixed with the remaining ingredients described in Table 1 in a 1 L tumble mixer (Turbula). Capsules are filled with this composition using a manual filling machine.
[0080] The bulk and tap densities of the granules and final compositions were evaluated using the methods described above. Flowability was also evaluated by testing the flow rate thro...
Embodiment 2
[0086] For the preparation of low-dose capsules, two variants of the batch A formulation were prepared on laboratory scale. Two batches of low dose capsules were prepared using the composition shown in Table 2 below. First, API, dicalcium phosphate, microcrystalline cellulose, croscarmellose sodium, and povidone were mixed in a V-shaped mixer in the amounts shown in Table 3 below. The API used in these examples is 2,5-dichloro-3-(5-(3,4-dihydroxy-5-nitrophenyl)-1,2,4-oxadiazol-3-yl )-4,6-lutidine 1-oxide. Purified water was added to the mixture and the mixture was manually mixed and granulated.
[0087] The granules were then dried in a cabinet dryer at 50°C for approximately 300 minutes. The dried granules are sieved. The sieved granules were then mixed with the rest of the croscarmellose sodium and colloidal silicon dioxide hydrate described in Table 3 in a V-blender. Magnesium stearate and talc are then added and mixed. Capsules are filled with this composition using ...
Embodiment 3
[0095] Three batches of pilot scale capsules with different doses were prepared using the composition shown in Table 3 below. Lot H is the low dose capsules, Lot J is the middle dose capsules and Lot L is the high dose capsules.
[0096] First, API, dicalcium phosphate, microcrystalline cellulose, croscarmellose sodium, and povidone were mixed in a high shear mixer granulator in the amounts shown in Table 3 below. The API used in these examples is 2,5-dichloro-3-(5-(3,4-dihydroxy-5-nitrophenyl)-1,2,4-oxadiazol-3-yl )-4,6-lutidine 1-oxide. Purified water was added to the mixture and the mixture was mixed in a high shear mixer granulator.
[0097] The granules are then dried in a fluid bed drier. The dried granules are sieved. The sieved granules were then mixed with the rest of the croscarmellose sodium and colloidal silicon dioxide hydrate described in Table 3 in a V-blender. Magnesium stearate and talc are then added and mixed. Capsules are filled with this composition ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com