Synthetic method of menthofuran
A synthesis method, the technology of mentha furan, which is applied in the field of synthesis of mentha furan, achieves the effects of improving the three wastes and operating conditions, mild reaction conditions, and simple post-treatment and purification processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
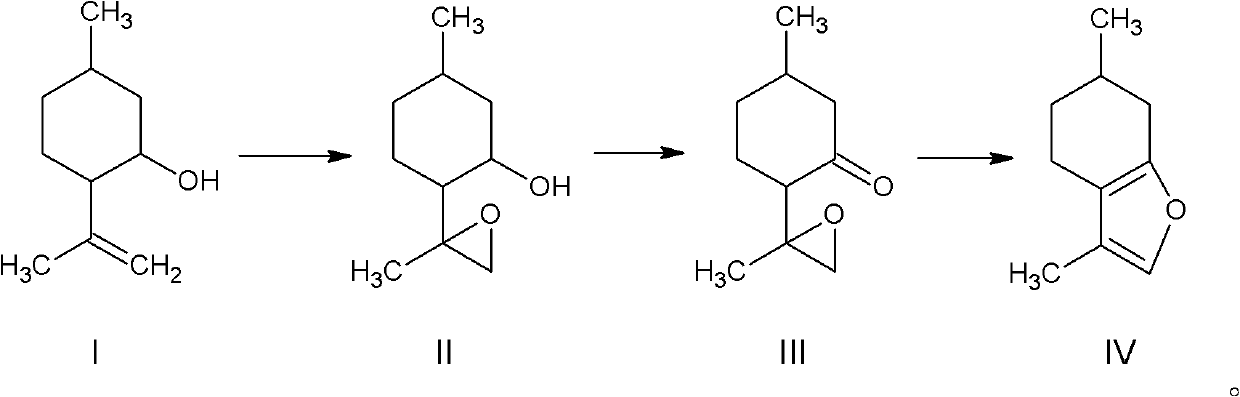
[0033] 1) Synthesis of isopulegol epoxide
[0034] Install an electric stirring device on a 2000ml four-necked bottle and place it in a water bath, and install a thermometer on a constant-pressure separating funnel and place it in an ice-water bath.
[0035] Add 900ml of acetone, 1g of manganese sulfate, 31g of acetonitrile, 185g of isopulegol, and 30g of anhydrous sodium carbonate into a 2000ml four-necked bottle under stirring; Add 516g of 30% hydrogen peroxide dropwise into a 2000ml four-neck bottle with a liquid funnel, keep the temperature below 35°C, and finish the dropwise addition in about 4 hours. Continue to stir for 1 hour. The reaction is complete by gas chromatography; the potassium iodide-starch test paper detects no discoloration. The solvent was evaporated under normal pressure, the lower water phase was let stand, the oil phase was washed with water, and the oil pump was distilled under reduced pressure to obtain 167.5 g of isopulegol epoxide (94-104° C. / 600 P...
Embodiment 2
[0041] 1) Synthesis of isopulegol epoxide
[0042] Install an electric stirring device on the 500ml four-necked bottle and place it in a water bath, and install a thermometer on a constant-pressure separating funnel and place it in an ice-water bath.
[0043]Add 200ml acetone, 15ml aqueous solution of 0.8g manganese sulfate, 10g acetonitrile, 55g isopulegol, and 8g sodium bicarbonate to a 500ml four-necked bottle with stirring; add 150g 28% hydrogen peroxide dropwise, and keep it below 38°C for about 3 hours After the dropwise addition, continue to stir for 1 hour; the potassium iodide-starch test paper detects no discoloration. The solvent was evaporated under normal pressure, the lower water phase was let stand, the oil phase was washed with water, and the oil pump decompressed distillation to obtain 49.2g of isopulegol epoxide (94-104°C / 600Pa); the yield was 81%.
[0044] 2) Synthesis of isopulegone epoxide
[0045] Install an electric stirring device on a 1000ml three-ne...
Embodiment 3
[0049] 1) Synthesis of isopulegol epoxide
[0050] Install an electric stirring device on the 500ml four-necked bottle and place it in a water bath, and install a thermometer on a constant-pressure separating funnel and place it in an ice-water bath.
[0051] Add 200ml of methanol, 0.6g of manganese sulfate, 9g of acetonitrile, 47g of isopulegol, and 8g of sodium bicarbonate into a 500ml four-necked bottle under stirring; Add 130g of 30% hydrogen peroxide dropwise into a 2000ml four-necked bottle with a liquid funnel, keep the temperature below 38°C, complete the dropwise addition in about 3 hours, and continue stirring for 1 hour; the potassium iodide-starch test paper did not change the color. The solvent was distilled off under normal pressure, the lower water phase was let stand to release, the oil phase was washed with water, and the oil pump decompressed distillation to obtain 41g of isopulegol epoxide (94-104°C / 600Pa); the yield was 79%.
[0052] 2) Synthesis of isopul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com