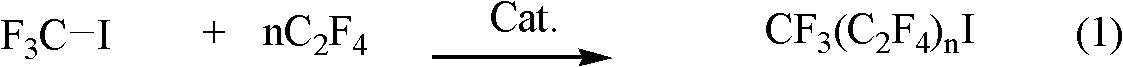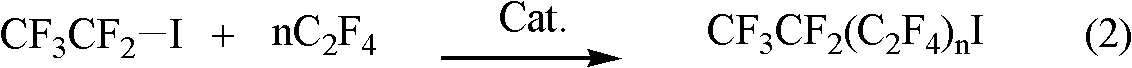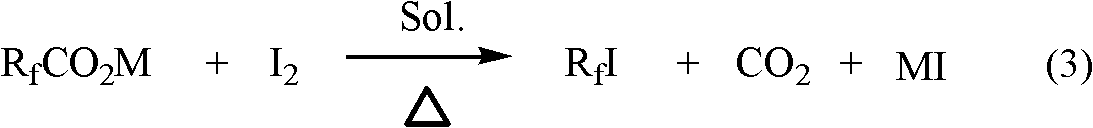Preparation method for perfluoroalkyl iodide with low carbon number
A technology of perfluoroalkyl iodide and perfluoroiodoalkane is applied in the field of preparation of low carbon number perfluoroiodoalkane, can solve the problems of large amount of reaction solvent, poor selectivity, long production cycle and the like, and is beneficial to industrialization Amplification, yield selectivity improvement, thermal decomposition temperature reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1 (trifluoroiodomethane preparation)
[0060] Put 887 g CF 3 CO 2 K, 1440 grams of elemental iodine and 12.9 grams of anhydrous silver trifluoroacetate, heated the reaction mixture under stirring, and when the internal temperature was 150 to 160° C., the reaction proceeded steadily for 3 hours. The crude product was collected in a cold trap (-70~-80° C.), and further purified by rectification to obtain 911 g of iodotrifluoromethane product with a GC content of 99.1% and a yield of 82%.
[0061] The structural identification data of trifluoroiodomethane are as follows:
[0062] MS (M r =196), m / z(%relative intensity), EI: 50(10), 69(88), 127(100), 177(30), 196(85).
[0063] 13 C NMR (100MHz, CDCl 3 ): δ79.185(q, CF 3 , J CF =344Hz)ppm
[0064] 19 F NMR (376.4MHz, CDCl 3 ): δ-5.200(s, CF3 )ppm
[0065] When the temperature of the reaction residue is lowered below 100°C, adding 500ml of water can completely dissolve the solid residue, which is used t...
Embodiment 2
[0066] Embodiment 2 (trifluoroiodomethane preparation)
[0067] Put 1025 g CF 3 CO 2 Na, 1932 grams of elemental iodine and 16.7 grams of silver trifluoroacetate, heated the reaction mixture under stirring, and when the internal temperature was 170~180° C., the reaction proceeded smoothly for 4 hours. The crude product was collected in a cold trap (-70 to -80° C.), and further purified by distillation to obtain 1108 grams of product with a GC content of 99.3% and a yield of 75%.
[0068] When the temperature of the reaction residue is lowered below 100°C, adding 700ml of water can completely dissolve the solid residue, which is used to recover iodine in the aqueous solution. Add 2700ml of 30% hydrogen peroxide to the above-mentioned iodide-containing aqueous solution, react at 40-60°C for 6 hours, convert iodide into elemental iodine, and then distill and remove water under reduced pressure to obtain crude iodine elemental substance. The iodine content in the obtained crude...
Embodiment 3
[0069] Embodiment 3 (trifluoroiodomethane preparation)
[0070] Take 6.2 grams of silver oxide and add it to 1612ml of trifluoroacetic acid under mechanical stirring; take 1500 grams of potassium hydroxide solid and dissolve it in 1100ml of water to form an aqueous solution of potassium hydroxide, then add the aqueous solution dropwise to the above-mentioned In the mixed solution, adjust the pH value to 7. The obtained potassium trifluoroacetate (silver) aqueous solution is vacuum-dried and dehydrated at a temperature of 800-110° C. to obtain 3140 grams of potassium trifluoroacetate containing a small amount of silver trifluoroacetate. Store spare. The yield obtained was 95%.
[0071] Put 920 grams of above-mentioned potassium trifluoroacetate containing a small amount of silver trifluoroacetate in a 3-liter there-necked bottle equipped with agitator, thermometer, reflux condenser (safety bottle, gas scrubber and cold trap connected in series behind the condenser) With 1540 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com