Novel steroid compound, and preparation method and application thereof
A technology of steroidal compounds and compounds, applied in the direction of steroidal compounds and organic chemistry, can solve the problems of high total cost and low yield of addition reaction, and achieve the effects of reduced production cost, easy availability of raw materials, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
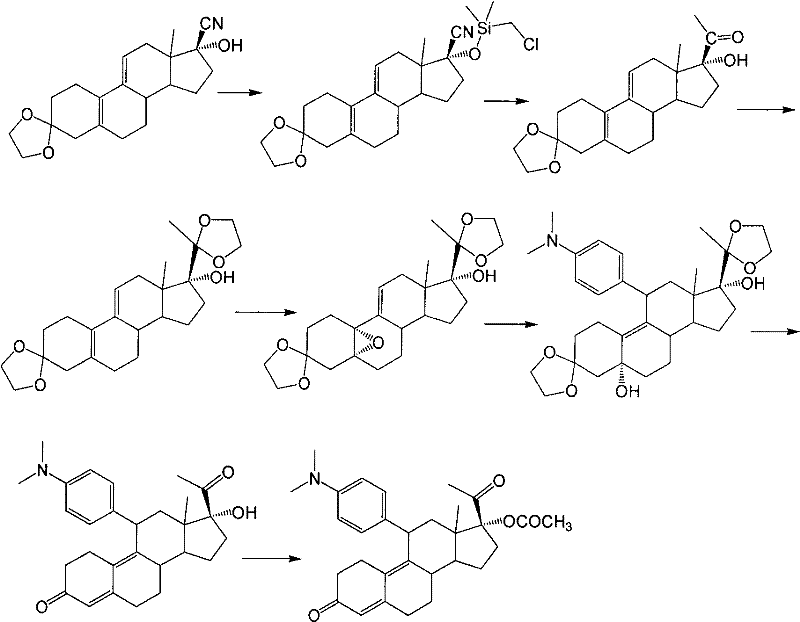
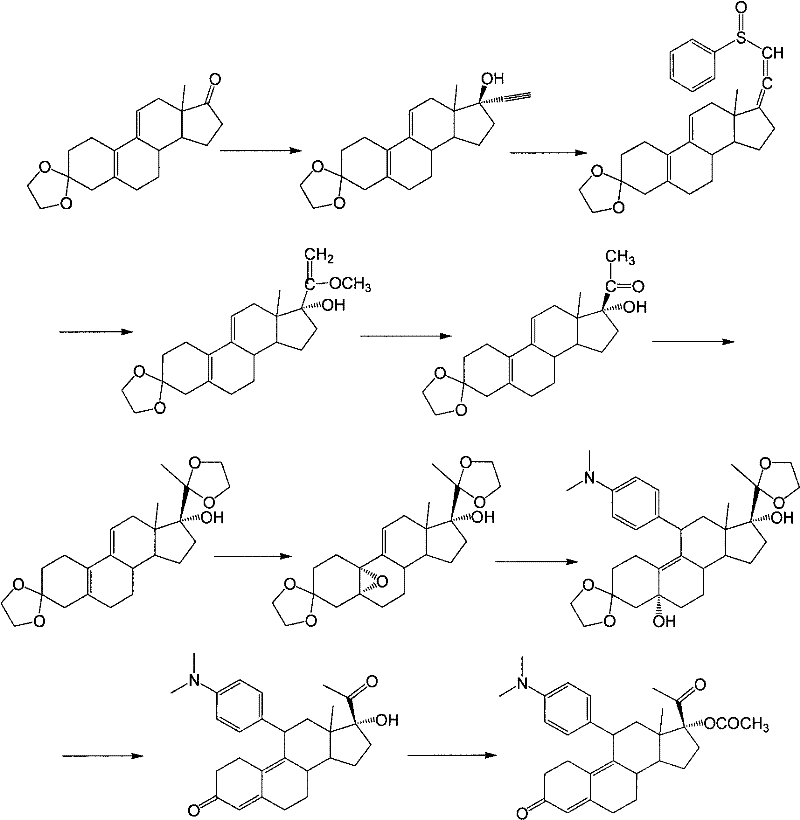
[0029] Example 1: 3-(ethylenedioxy)-17α-ethynyl-17β-hydroxy-estro-5(10),9(11)-diene (compound 2)
[0030] Under the protection of nitrogen, add 1.2 L of tetrahydrofuran and 400 g of potassium hydroxide into the dry reaction flask, then lower the temperature to below 5° C., and pass through acetylene gas. Add 1L of acetone, raise the temperature to 35°C-40°C, keep flowing acetylene gas, and stir for 2 hours. 100 g of compound 1 was dissolved in 1 L of tetrahydrofuran, and added dropwise to the reaction solution. After the dropwise addition, the reaction was controlled at 35° C. to 40° C. for 1 hour. After the reaction was completed, 750 ml of saturated ammonium chloride solution was added and the reaction mixture was stirred for 10 min. The organic layer was separated, and the aqueous layer was extracted with 300 ml of tetrahydrofuran. The combined organic layers were washed with 750 ml of saturated ammonium chloride, concentrated to 600 ml under reduced pressure, poured into...
Embodiment 2
[0032] Example 2: 3-(ethylenedioxy)-17α-ethynyl-17β-hydroxy-5α,10α-epoxy-estro-9(11)-ene (compound 3)
[0033] Add 735ml of dichloromethane, 105g of compound 2 and 21g of disodium hydrogen phosphate into the reaction bottle, after cooling to 0-5°C, add 45ml of hexafluoroacetone and 95ml of 30% hydrogen peroxide, and keep stirring for 16 hours. Then, 590 ml of an aqueous solution of 10% sodium thiosulfate was added, and stirred for 30 minutes. The organic layer was separated, the aqueous layer was extracted with dichloromethane, the organic layers were combined, washed with saturated sodium bicarbonate solution and water successively, dried with anhydrous sodium sulfate and concentrated to dryness under reduced pressure to obtain compound 3. The resulting product was used directly in the next step without purification.
Embodiment 3
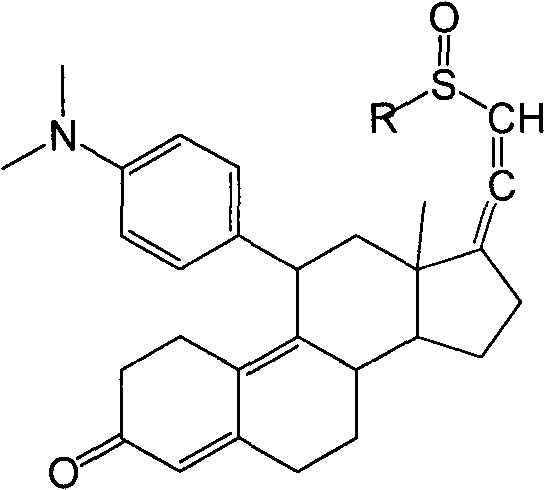
[0034] Example 3: 3-(ethylenedioxy)-11β-[4-(N,N-dimethyl-amino)-phenyl]-17α-ethynyl-17β,5α-dihydroxy-estro- 9(11)-ene (Compound 4)
[0035] Under nitrogen protection, 210ml of tetrahydrofuran, 23.8g of magnesium chips, 0.3 iodine and 10mL of 4-bromo-N,N-dimethylaniline in tetrahydrofuran (180g of 4-bromo-N,N-dimethyl Aniline is dissolved in 900mL THF); the temperature is raised to reflux to initiate the reaction, the temperature is lowered to 40°C to 50°C, and the remaining 4-bromo-N,N-dimethylaniline tetrahydrofuran solution is added dropwise at this temperature, and the temperature is kept at 40°C React at ~50°C for 2 hours to obtain 4-bromo-N,N-dimethylaniline Grignard reagent. Cool down to 20°C to 30°C, add 5g cuprous chloride, keep the temperature, slowly add 3-ketal tetrahydrofuran solution (the above compound 3 is dissolved in 500mL tetrahydrofuran), after the dropwise addition, continue to cool at 20°C to 30°C. ℃ reaction 2h. After the reaction was completed, 600 ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com