Diltiazem hydrochloride delayed release bilayer osmotic pump controlled released tablet and its preparation method
A controlled-release technology of diltiazem hydrochloride and osmotic pumps, which is applied in the fields of pharmaceutical formulations, medical preparations of non-active ingredients, pill delivery, etc., and can solve the problems of low blood drug concentration, short maintenance time of blood drug concentration, and no chronological rhythm of blood pressure fluctuations. Pertinence and other issues to achieve the effect of maintaining blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
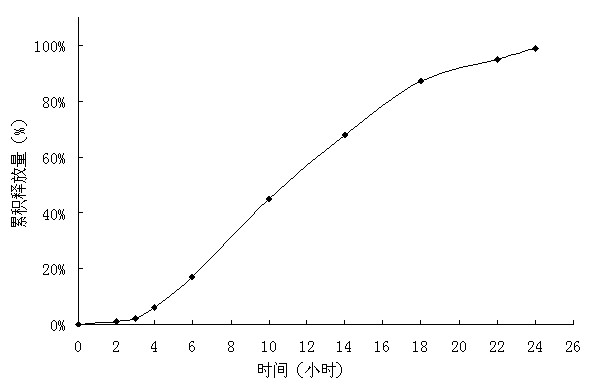
Embodiment 1
[0030] Tablet prescription:
[0031]
[0032] Preparation Process:
[0033] Pass the prescribed amount of medicine, polyoxyethylene with a molecular weight of 200,000, and mannitol through a 80-mesh sieve, mix evenly, use absolute ethanol as a binder to make a soft material, granulate with a 20-mesh sieve, and dry the wet granules through a 18-mesh sieve Granules, dry granules and lubricants are mixed to obtain the drug layer, and the same method will obtain the booster layer. The tablet core is obtained by compressing the two layers of auxiliary materials twice. Dissolve polyvinyl alcohol and polyethylene glycol with an average molecular weight of 10,000 in a mixed solvent of acetone and water and disperse evenly; dissolve cellulose acetate and polyethylene glycol with an average molecular weight of 4,000 in acetone and water respectively and mix evenly . Use a coating pan to first coat the tablet core with an isolation coat with a weight gain of 20%, and then coat the ...
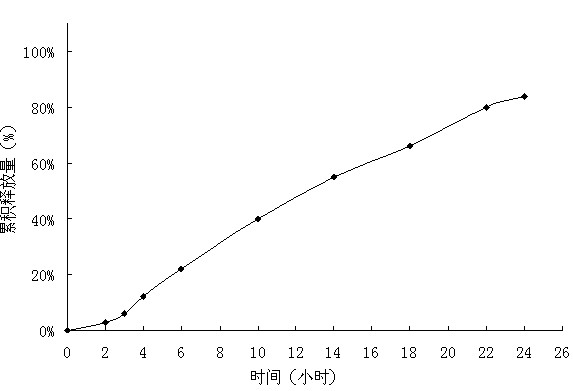
Embodiment 2
[0035] Tablet prescription:
[0036]
[0037] Preparation Process:
[0038] Pass the prescribed amount of medicine, polyoxyethylene with a molecular weight of 200,000, and sodium chloride through an 80-mesh sieve, mix evenly, use absolute ethanol as a binder to make a soft material, and granulate with a 20-mesh sieve. After the wet granules are dried, the 18-mesh Sieve the granules, and mix the dry granules with a lubricant to obtain the drug layer. The same method will obtain the booster layer. The tablet core is obtained by compressing the two layers of auxiliary materials twice. Dissolve polyvinylpyrrolidone and polyethylene glycol with an average molecular weight of 10,000 in a mixed solvent of acetone and water and disperse evenly; dissolve cellulose acetate and polyethylene glycol with an average molecular weight of 4,000 in acetone and water and mix evenly . Use a coating pan to first coat the tablet core with an isolation coat with a weight gain of 20%, and then ...
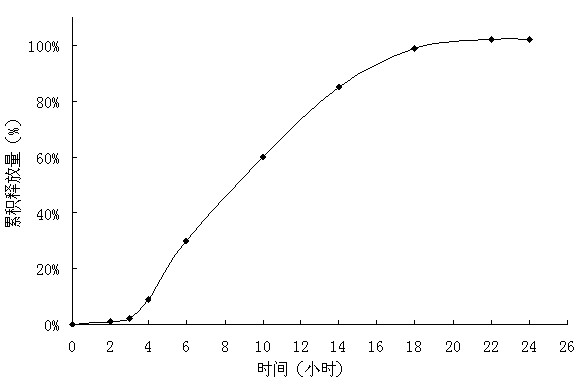
Embodiment 3
[0040] Tablet prescription:
[0041]
[0042] Preparation Process:
[0043]Pass the prescribed amount of medicine, polyoxyethylene with a molecular weight of 200,000, and sodium chloride through an 80-mesh sieve, mix evenly, use absolute ethanol as a binder to make a soft material, and granulate with a 20-mesh sieve. After the wet granules are dried, the 18-mesh Sieve the granules, and mix the dry granules with a lubricant to obtain the drug layer. The same method will obtain the booster layer. The tablet core is obtained by compressing the two layers of auxiliary materials twice. Dissolve hypromellose and polyethylene glycol with an average molecular weight of 10,000 in a mixed solvent of acetone and water and disperse evenly; dissolve cellulose acetate and polyethylene glycol with an average molecular weight of 4,000 in acetone and water and well mixed. Use a coating pan to first coat the tablet core with an isolation coat with a weight gain of 20%, and then coat the o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com