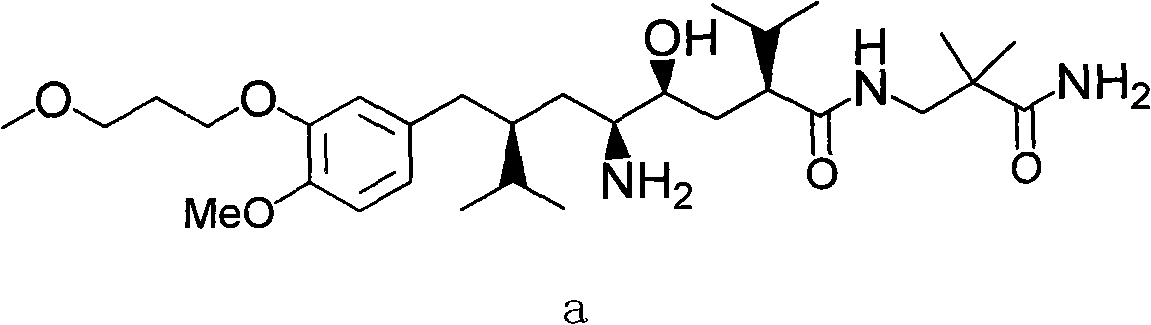
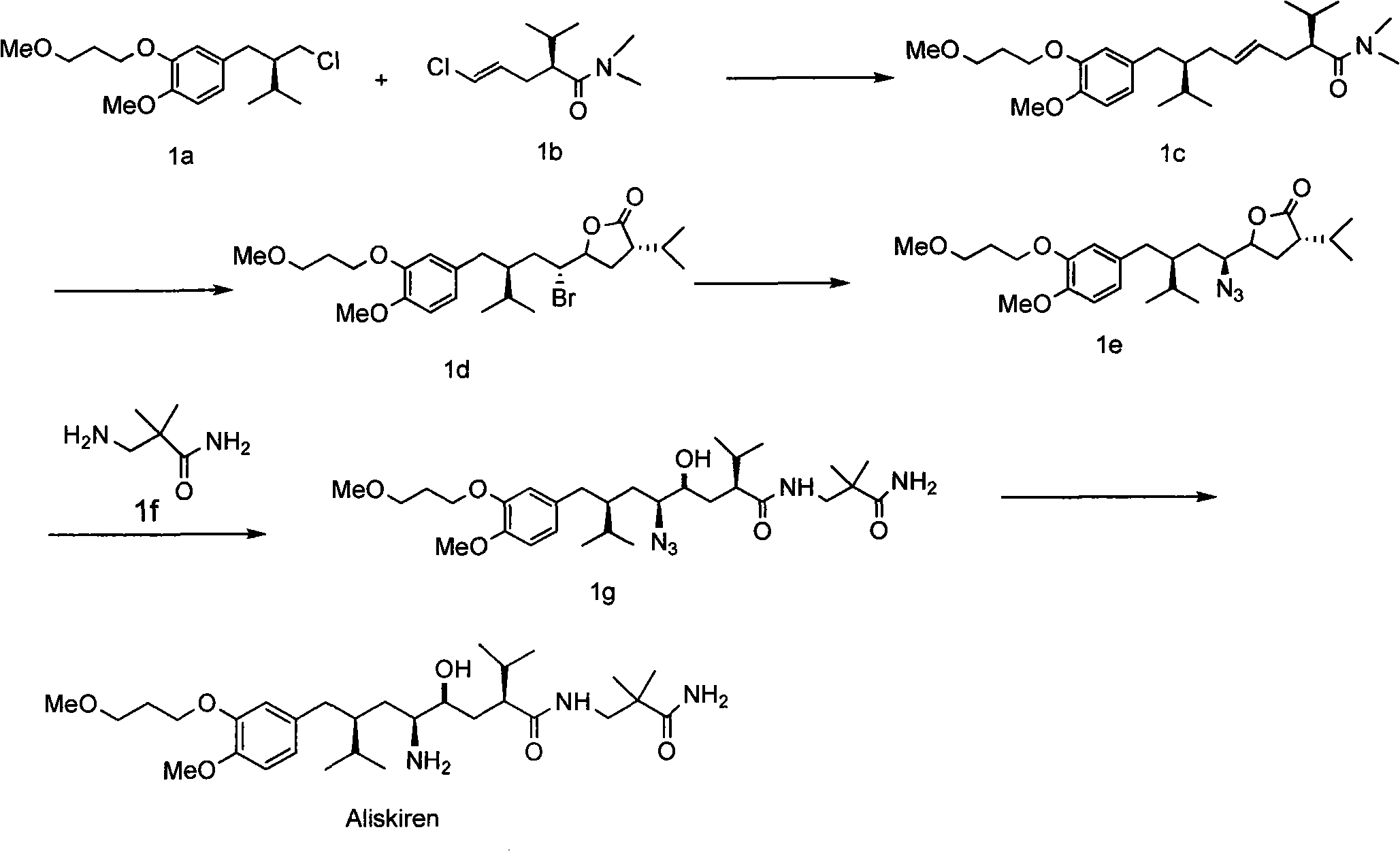
Aliskiren, its intermediates and medicinal salt, as well as preparation method thereof
A technology of intermediates and compounds, applied in the field of pharmaceutical synthesis, can solve the problems of difficult product separation and purification, high cost of reaction raw materials, low yield and purity, etc., and achieve the effect of easy crystallization, meeting industrial production and improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
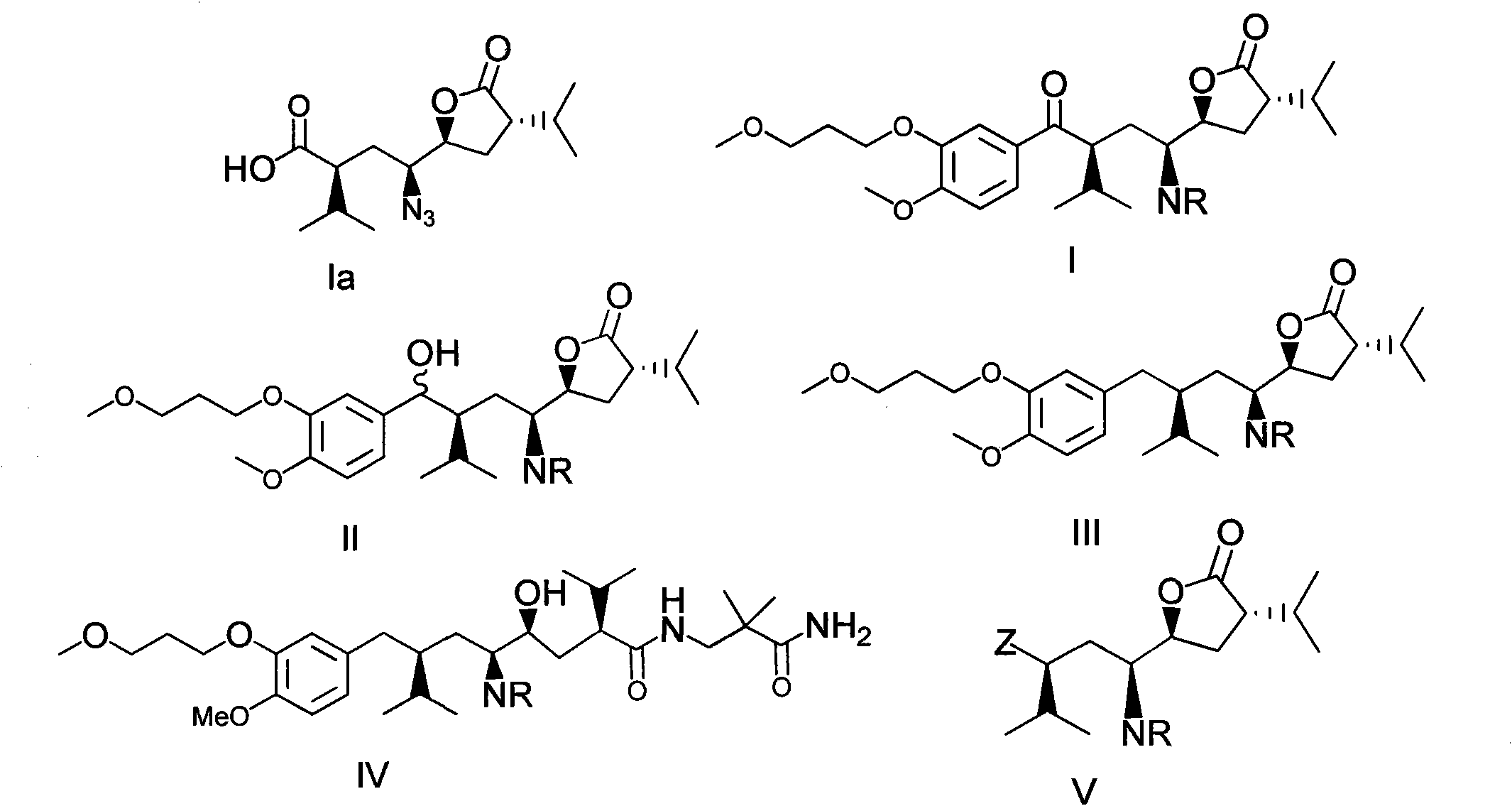
[0047] Example 1 Formula III 1 compound synthesis
[0048]
[0049] According to the method disclosed in patent WO2007045420A, the product Ia was synthesized as a synthetic raw material.
[0050] 1) Compound V 1 Synthesis
[0051]
[0052] in N 2Under protection, 26.45g (88.9mmol) of compound Ia was dissolved in 150mL of toluene, and 7.2ml of thionyl chloride and 0.5ml of DMF were slowly added dropwise at room temperature. The temperature rose to 37°C, heated to reflux for 2 hours after the dropwise addition, then lowered to room temperature, added 10ml of methanol dropwise, and stirred for 2 hours to obtain V 1 , the product does not need to be separated, and the next reaction is continued.
[0053] 2) by the azide compound V 1 Preparation of Amino Compound V by Pa / C Catalytic Hydrogenation 2
[0054]
[0055] The above obtained V 1 Move into the hydrogenation reactor, add 5g of catalyst Pd / C, react at normal temperature and pressure for 21 hours, the reac...
Embodiment 2
[0068] Example 2 Formula III 1 compound synthesis
[0069] According to the method disclosed in patent WO2007045420A, Ia was synthesized as a synthetic raw material.
[0070] 1) Preparation of Amino Compound Ib by Pa / C Catalytic Hydrogenation of Azide Compound Ia
[0071]
[0072] In the hydrogenation reactor, 26.45g of compound Ia was dissolved in 150ml of ethanol, 5g of 5% Pd / C catalyst was added, reacted at room temperature for 20 hours, then filtered, the filtrate was washed with water, and the organic phase was separated and concentrated to obtain compound Ib.
[0073] 2) Compound Ib reacts with phthaloyl chloride to synthesize amino-protected compound Ic 1
[0074]
[0075] Under the protection of nitrogen, 18.57g of compound Ib was dissolved in 95ml of tetrahydrofuran, the temperature was controlled to 0°C, 3.7g of triethylamine was added under stirring, and then 12.2g of phthaloyl chloride-THF solution was slowly added dropwise, maintaining the same temperat...
Embodiment 3
[0087] Example 3 Formula IV 1 compound synthesis
[0088]
[0089] Under nitrogen protection, add 150g (227mmol) III 1Compound and 80g (690mmol) compound BP-a, then add 111ml (79mmol) triethylamine, drop into 3.1g (32mmol) 2-hydroxypyridine, the reaction mixture is heated to 80 ℃, stirring reaction 24 hours, TLC detection reaction is complete, The reaction solution was diluted with saturated sodium bicarbonate, then extracted with ethyl acetate, the organic phases were combined, washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, filtered with suction, and the filtrate was concentrated under reduced pressure to obtain a light yellow foamy substance, iso Recrystallized from propyl ether to obtain white solid substance IV 1 .
[0090] IV 1 :
[0091] H-NMR (400MHz, CDCl3): 0.86(q, 6H), 0.93(d, 3H), 1.01(d, 3H), 1.38(d, 6H), 1.96-2.00(m, 2H), 2.10(m, 2H), 2.15(m, 2H), 2.42(m, 2H), 2.81(m, 2H), 3.28(s, 3H), 3.28-3.81(m, 3H), 3.94(s, 3H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com