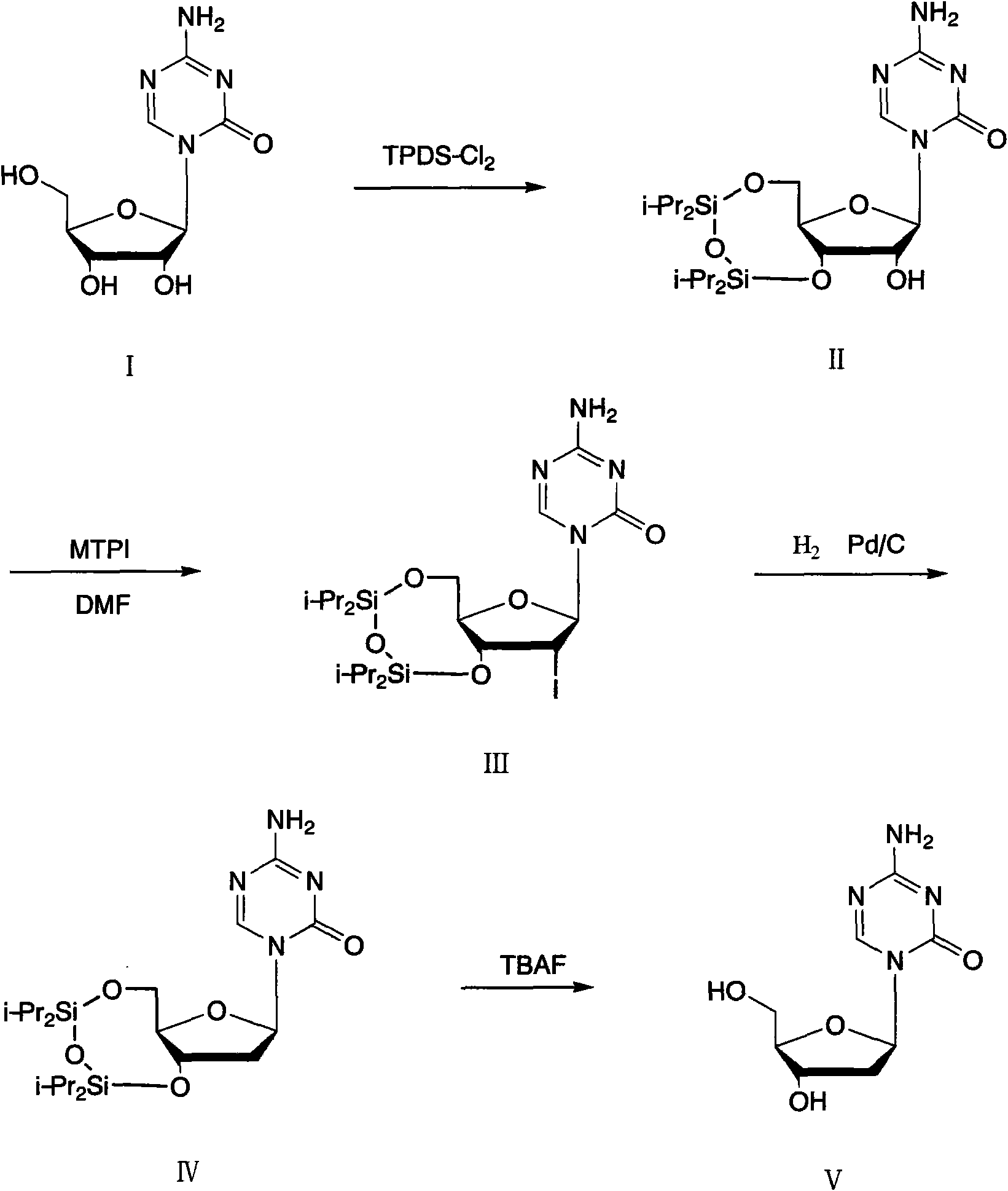
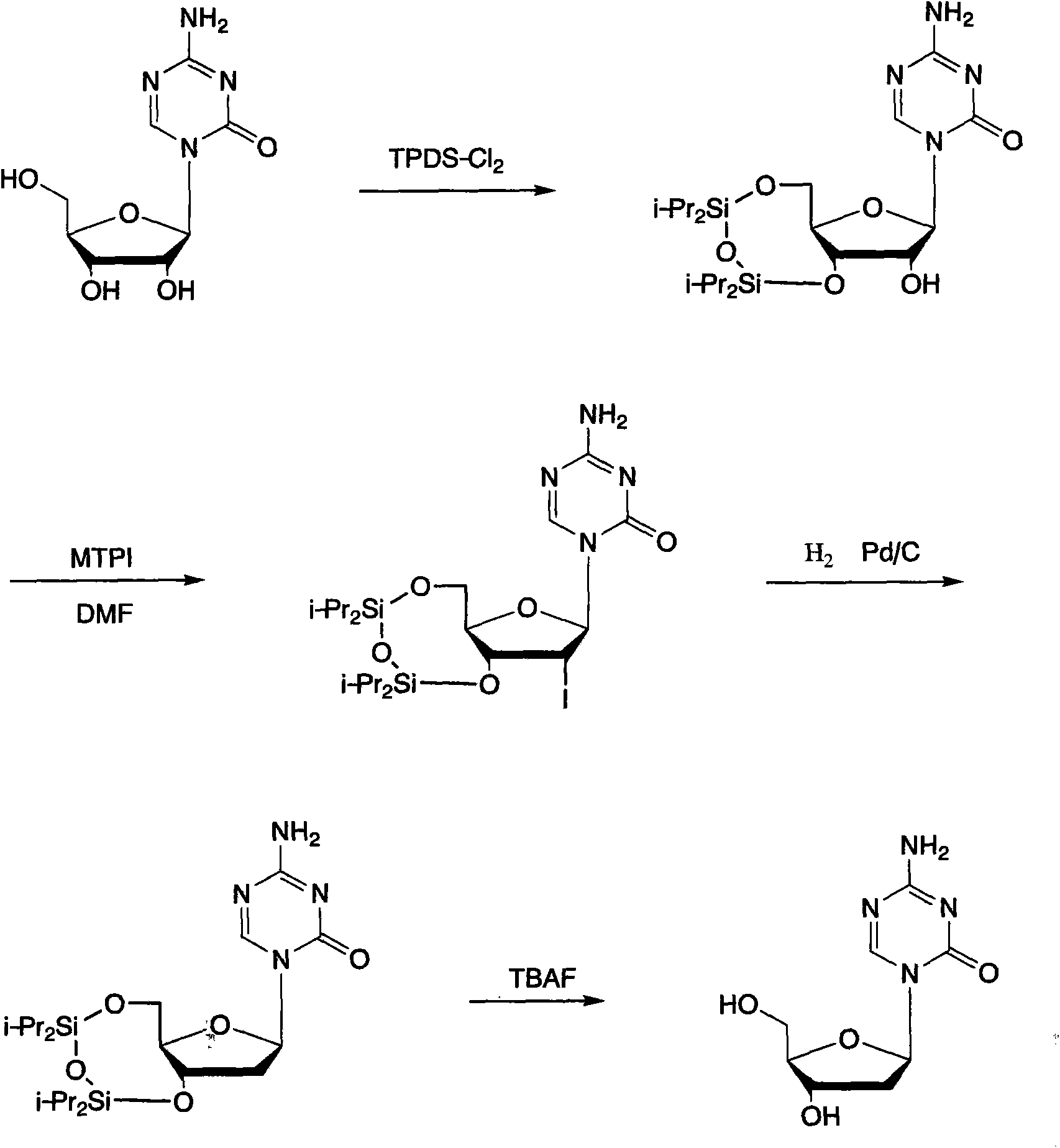
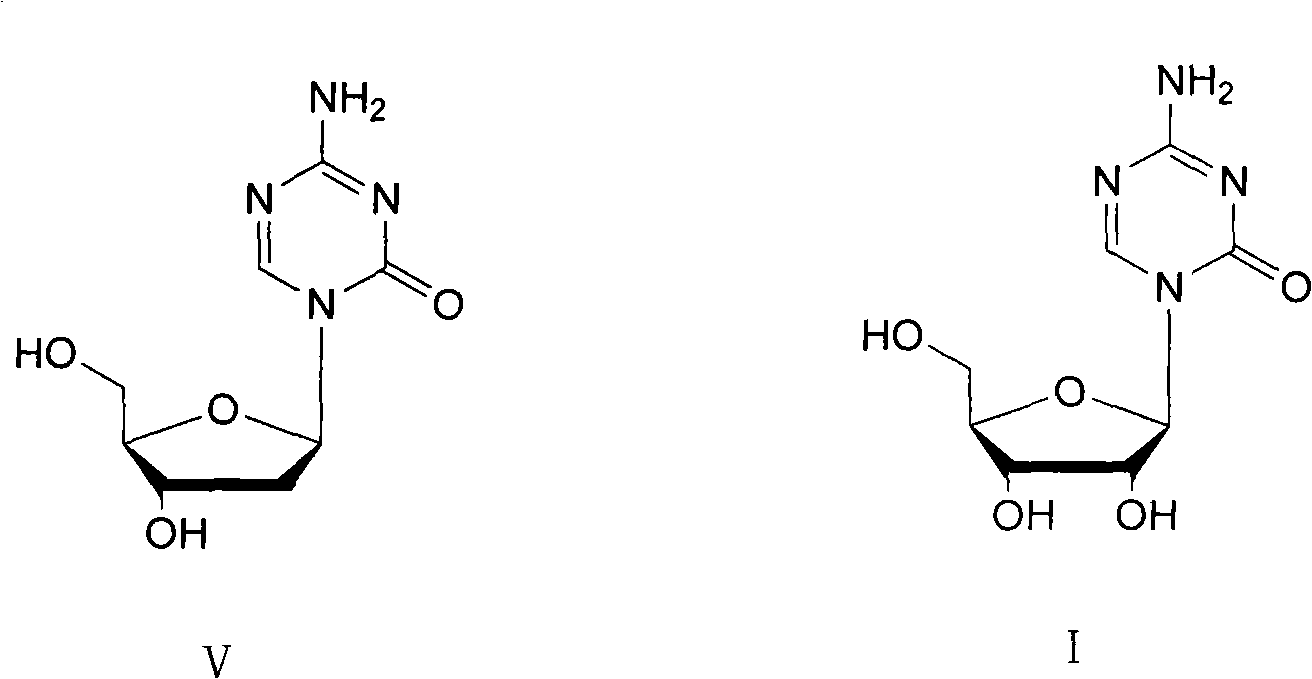
Method for preparing decitabine
A technology of citabine and methanol, applied in the field of chemical pharmaceuticals, can solve the problems of cumbersome industrial operation, low value of large-scale production, expensive protective agent, etc., and achieve the effect of simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the preparation of II
[0027] 12.2g I, 17.8g TPDS-Cl 2 Put 100ml of pyridine into a four-necked bottle, start stirring, and react at 25-30°C for 5 hours. After the reaction, concentrate the reactant to dryness, slowly add 100ml of ethyl acetate, stir to dissolve, add 30ml of dilute hydrochloric acid, 30ml of water , 30mlNaHCO 3 Wash the organic layer with anhydrous Na 2 SO 4 Dry, filter after drying, distill to dryness, add 100ml of ethanol to obtain 20.9g of product II after refining.
Embodiment 2
[0028] Embodiment 2: Preparation of III
[0029] Dissolve 14.6g of II and 20.3g of MTPI in 100ml of anhydrous DMF, add 10ml of methanol after stirring, and react at 25-30°C for 30min. After the reaction was completed, the reactant was distilled to dryness, added 100ml of chloroform and stirred to dissolve, washed with 30ml of water three times, and the organic layer was collected and washed with anhydrous Na 2 SO 4 After drying, distill to dryness after drying, and add ethanol to refine to obtain 15.4 g of product III.
Embodiment 3
[0030] Embodiment 3: Preparation of III
[0031] Dissolve 14.6g of II and 16.3g of MTPI in 100ml of anhydrous DMF, add 10ml of methanol after stirring, and react at 20-25°C for 1 hour. After the reaction was completed, the reactant was distilled to dryness, added 100ml of chloroform and stirred to dissolve, washed with 30ml of water three times, and the organic layer was collected and washed with anhydrous Na 2 SO 4 After drying, distill to dryness after drying, and add chloroform-n-hexane to refine to obtain 16.1 g of product III.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com