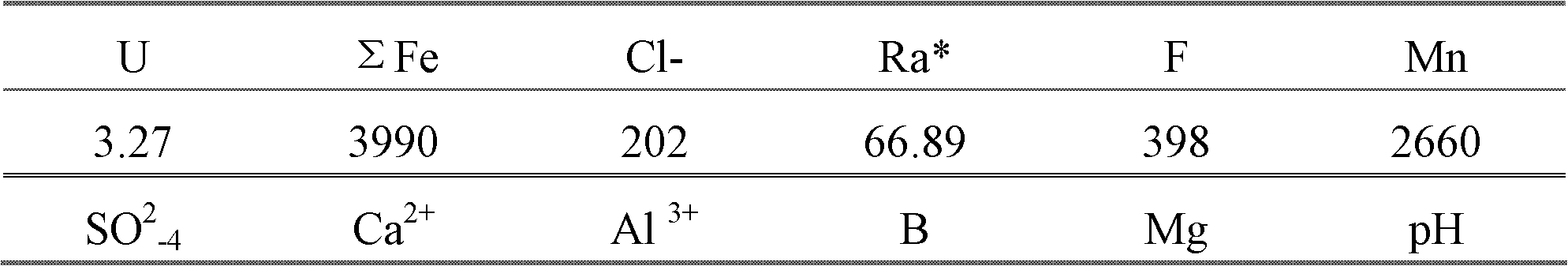Method for removing fluorine in acid uranium process wastewater
A process and acidic technology, which is applied in the field of fluorine removal in acid uranium process wastewater, can solve the problems such as difficult removal of fluorine ions, and achieve the effects of fast settling speed, saving reagents and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
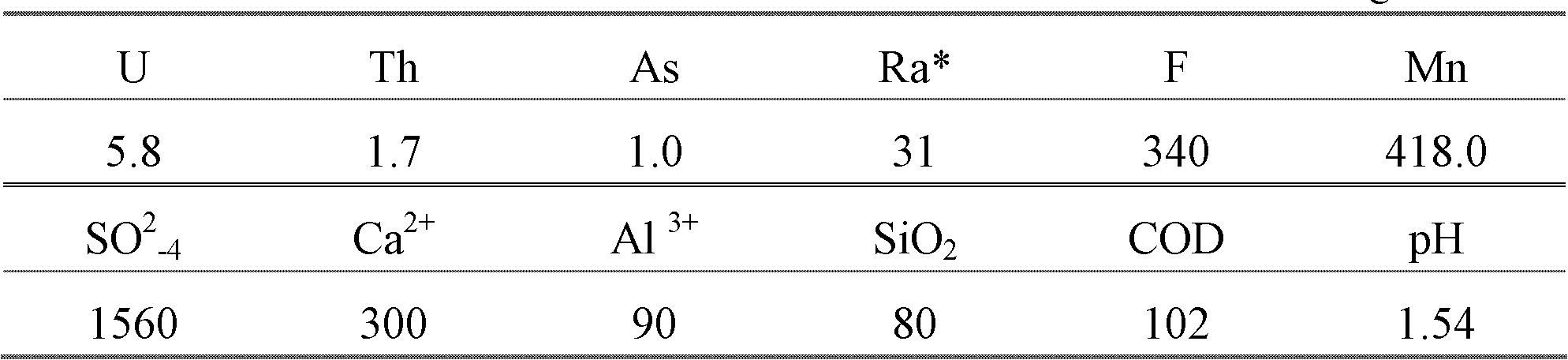
[0029] The acidic uranium process wastewater in the examples comes from the acidic industrial production wastewater of a uranium mine, and the analysis results of its composition are shown in the following table.
[0030] Table 1 Wastewater Composition mg / L
[0031]
[0032] *Note: Radium activity unit: Bq / L
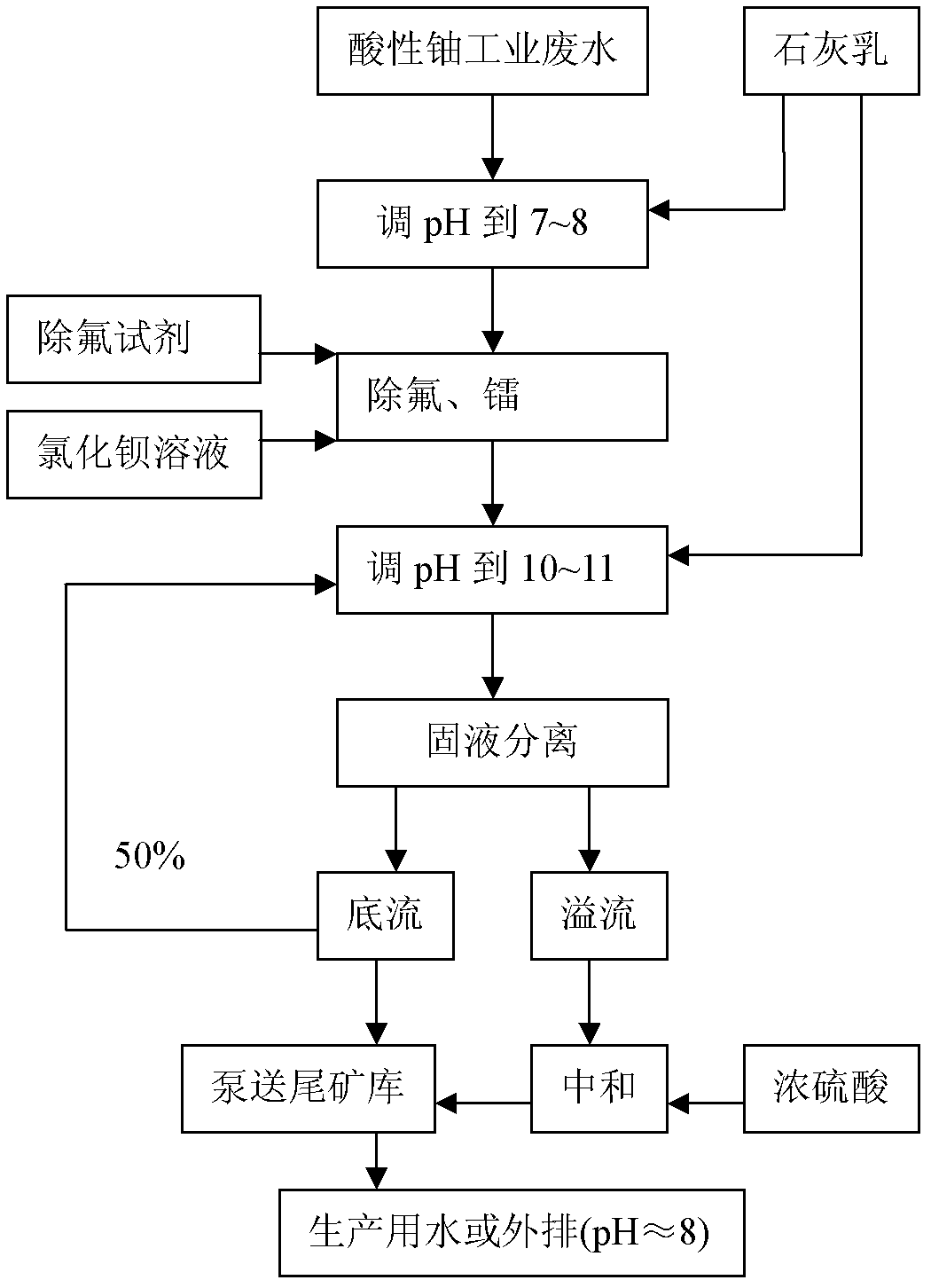
[0033] Such as figure 1 Shown, the removal method of fluorine in a kind of acid uranium process wastewater of the present invention, it comprises the steps:
[0034] (1) Add milk of lime Ca(OH) to acid uranium process wastewater 2 Neutralize the solution so that the pH of the solution is controlled at 7.5;
[0035] (2) sulfate radical concentration ρ(SO in the solution of step (1) gained 2- 4 )≥250mg / l, add BaCl 2 In addition to radium, add a fluorine removal reagent to remove fluorine, and then stir and react at 25°C for 45 minutes; the BaCl 2 The added mass is 10mg BaCl per liter of solution 2 The added quality of the defluorination reagent is 60 times of th...
Embodiment 2
[0044] The acidic uranium process wastewater in the examples comes from a certain uranium ore hydrometallurgy process wastewater, and its composition analysis results are shown in the table below.
[0045] Table 2 Wastewater Composition mg / l
[0046]
[0047]
[0048] *Note: Radium activity unit: Bq / l
[0049] A method for removing fluorine in acidic uranium process wastewater according to the present invention comprises the steps of:
[0050] (1) Add 15wt% Ca(OH) to acid uranium process wastewater 2 Neutralize the solution so that the pH of the solution is controlled at 8;
[0051] (2) sulfate radical concentration ρ(SO in the solution of step (1) gained 2- 4 )≥250mg / l, add BaCl 2 In addition to radium, add a fluoride removal reagent to remove fluorine, and then stir and react at 15°C for 30 minutes; the BaCl 2 The added quality is 20mg BaCl per liter of solution 2 The added quality of the defluorination reagent is 65 times of the mass concentration of fluorine i...
Embodiment 3
[0055] The acidic uranium process wastewater in the examples is derived from the uranium hydrometallurgy test process wastewater in a certain mine, and the analysis results of its composition are shown in the following table.
[0056] Table 3 Wastewater Composition mg / l
[0057]
[0058] *Note: Radium activity unit: Bq / l
[0059] A method for removing fluorine in acidic uranium process wastewater according to the present invention comprises the steps of:
[0060] (1) Add 10wt% Ca(OH) to acid uranium process wastewater 2 Neutralize the solution so that the pH of the solution is controlled at 7;
[0061] (2) solution sulfate radical concentration ρ(SO in step (1) gained 2- 4 ) ≥ 250mg / l add BaCl 2 In addition to radium, add a fluorine removal reagent to remove fluorine, and then stir and react at 5°C for 60 minutes; the BaCl 2 The added quality is 30mg BaCl per liter of solution 2 The addition quality of described fluorine removal reagent is 70 times of the mass concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com