High-efficiency superparamagnetic ferrite nano arsenic adsorbent and preparation process thereof
A superparamagnetic and preparation technology, which is applied in the direction of adsorption of water/sewage treatment, other chemical processes, alkali metal oxides/hydroxides, etc., can solve the problems of adsorbent recycling and harmful ions exceeding the standard, and achieve energy saving , low cost of raw materials, easy to control the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The method provided by the invention is used to prepare high-efficiency superparamagnetic magnesium ferrite nano-arsenic adsorbent, and its technological process is as follows:
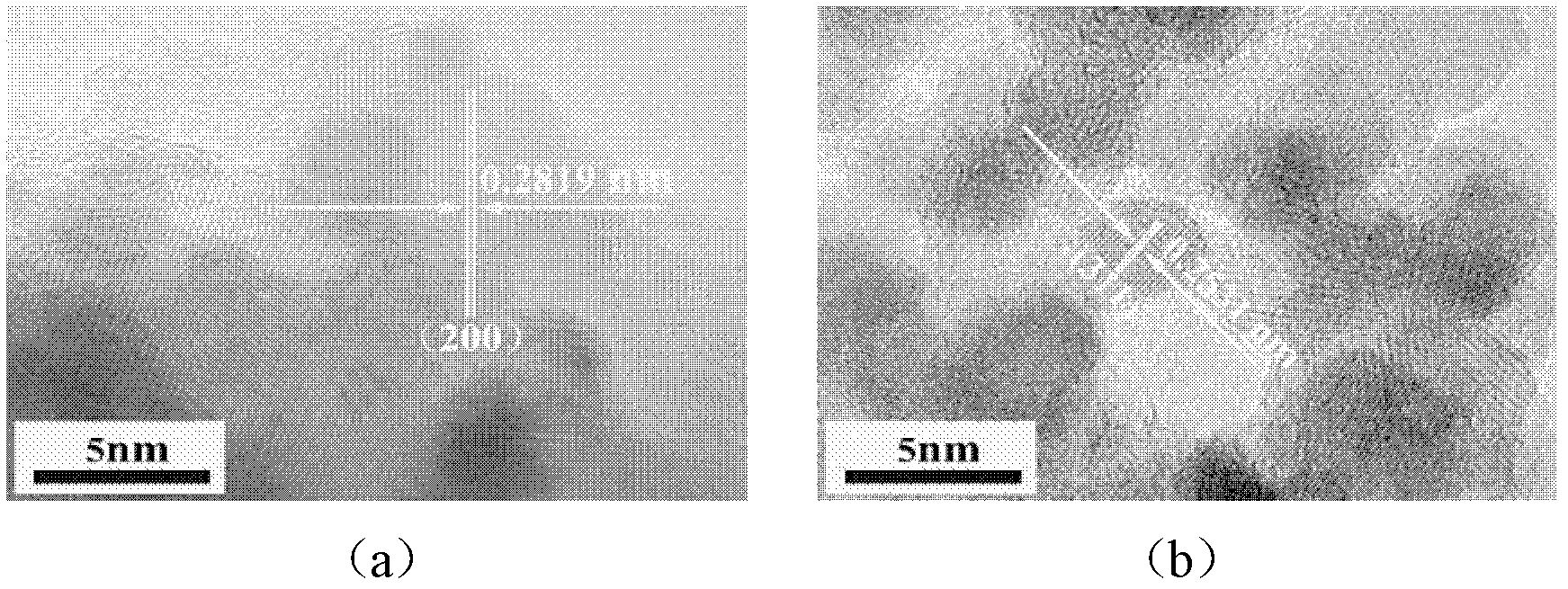
[0036] Under magnetic stirring, the anhydrous FeCl 3 (1.13g) and MgCl 2 (0.075g) is dissolved in dehydrated alcohol (80m1) and is configured into the chloride ethanol solution that molar concentration is 0.1M, then a certain amount of NaOH is slowly added in the chloride ethanol solution (the addition of sodium hydroxide=metal ion The valence state × the amount of metal chloride added, the two metal ions Mg 2+ with Fe 3+ Total after calculation respectively), fully mixed for 1 h under the action of magnetic stirring, and then introduced into a polytetrafluoroethylene reactor for solvothermal reaction. The temperature of the solvothermal reaction is 150 ° C, and the solvothermal time is 2 h. After the mixed solution was cooled to room temperature, it was centrifuged and ultrasonically washed....
Embodiment 2
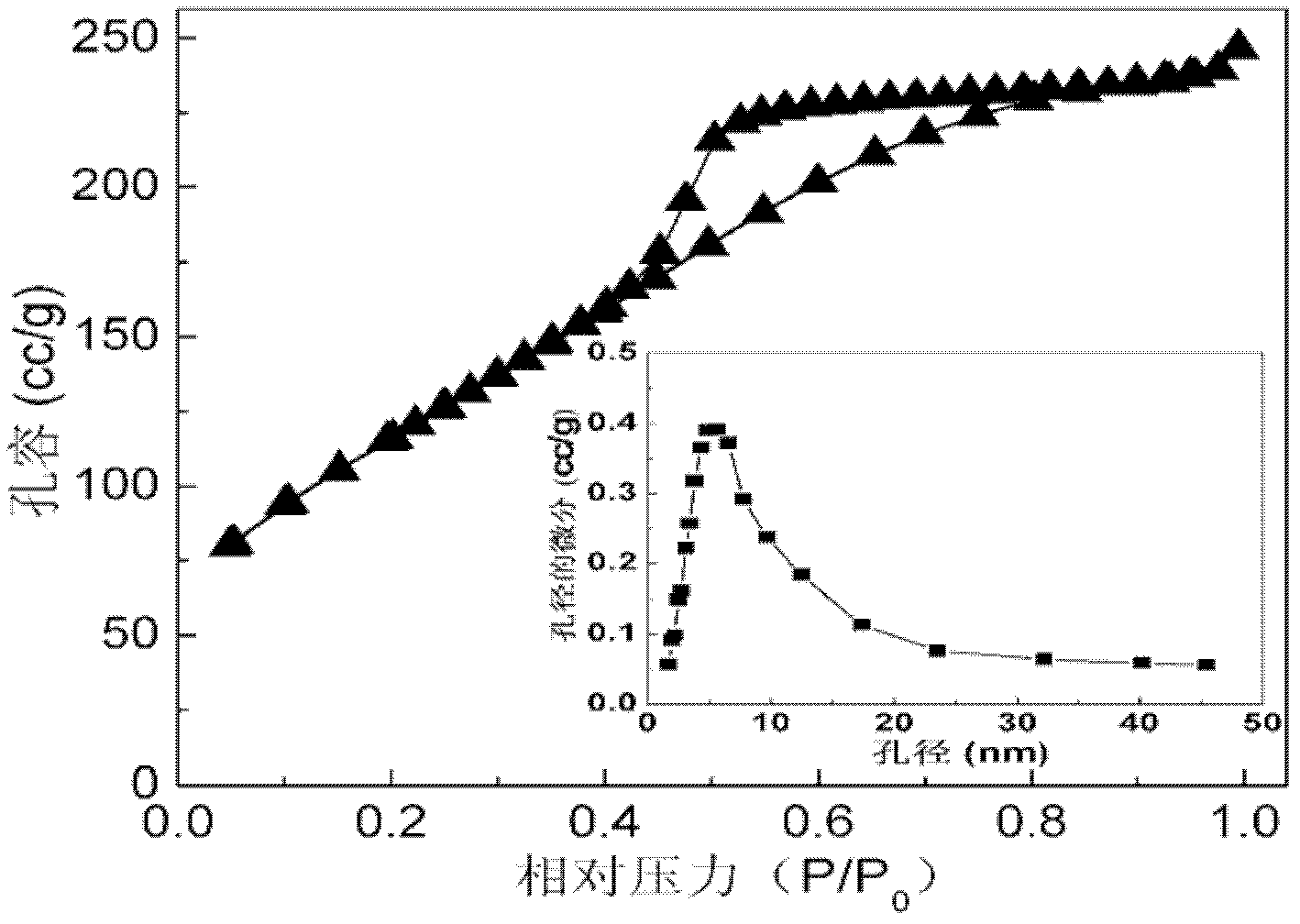
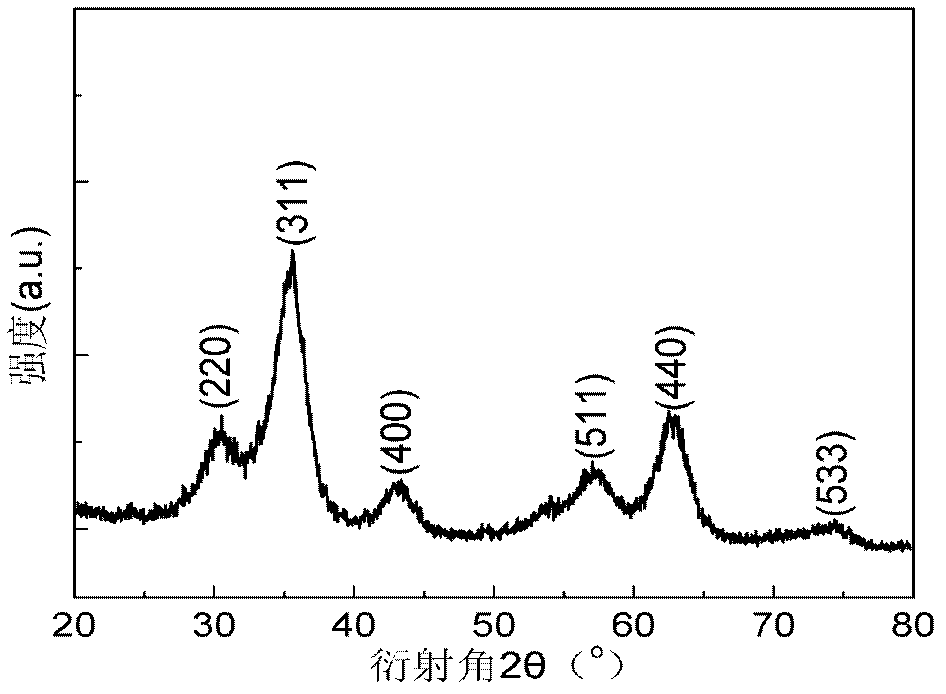
[0045] The difference from Example 1 is that the method provided by the present invention is used to prepare high-efficiency superparamagnetic manganese ferrite nano-arsenic adsorbent, and the prepared superparamagnetic nano-manganese ferrite powder is fully crystallized cubic spinel Mn 0.11 Fe 2.52 o 4 Phase, the specific surface area is 389m 2 / g, the particle size is 3-6nm.
[0046] When the adsorbent is used at 0.04g / L for one time, the adsorption efficiency of trivalent arsenic or pentavalent arsenic is 100%; the water sample after arsenic adsorption is magnetically separated to obtain nano-ferrite phase particles, and then washed with alkali Desorption and recycling, after 3-5 times of recycling, the adsorption efficiency of trivalent arsenic or pentavalent arsenic is 70-80%.
Embodiment 3
[0048] The difference from Example 1 is that the method provided by the present invention is used to prepare a high-efficiency superparamagnetic zinc ferrite nano-arsenic adsorbent, and the prepared superparamagnetic nano-zinc ferrite powder is a fully crystallized cubic spinel Zn 0.45 Fe 2.37 o 4 Phase, the specific surface area is 330m 2 / g, the particle size is 4-8nm.
[0049] When the adsorbent is used at 0.05g / L for one time, the adsorption efficiency of trivalent arsenic or pentavalent arsenic is 100%; the water sample after arsenic adsorption is magnetically separated to obtain nano-ferrite phase particles, and then washed with alkali Desorption and recycling, after 3-5 times of recycling, the adsorption efficiency of trivalent arsenic or pentavalent arsenic is 70-80%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Grain size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Average size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com