Sulfide heavy metal chelating agent and preparation method thereof
A heavy metal collector and sulfide technology, applied in chemical instruments and methods, water pollutants, organic chemistry, etc., can solve the problems of low yield, low zinc removal rate, long synthesis route, etc., and achieve short settling time. , Not easy to secondary pollution, easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
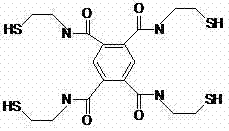
[0024] A kind of sulfide heavy metal collector N 1 ,N 2 ,N 4 ,N 5 -Tetrakis(2-mercaptoethyl) 1,2,4,5-pyromelliticamide, the preparation method of which is as follows:
[0025] At 20°C, in a 150mL three-neck flask, dissolve mercaptoethylamine hydrochloride to pyromellitic anhydride at a molar ratio of 4.4, that is, dissolve 5.0 g of mercaptoethylamine hydrochloride and 2.18 g of pyromellitic anhydride in 75 mL of dimethylformaldehyde In the amide, react under stirring for 4 hours to obtain the heavy metal chelating collector N 1 ,N 2 ,N 4 ,N 5 -Tetrakis(2-mercaptoethyl) 1,2,4,5-pyromellitic tetracarboxamide (TMBTC for short), the reaction yield is 22.43%.
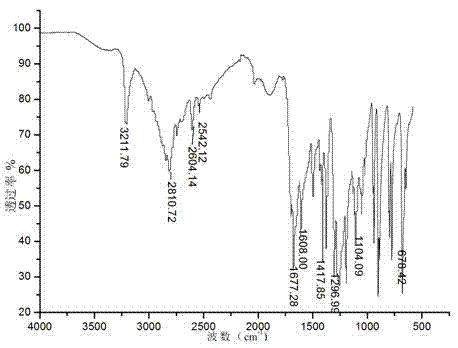
[0026] Infrared IR and nuclear magnetic NMR characterization results are as follows:
[0027] IR(KBr disk, cm -1 ): 3211.79 s(V N- H), 2542.12w( S-H ), 1677.28(V C=O), 1608 ss (δ in-plane bending N-H), 1417.85 m (V C=C), 1296.99ss (V C-N), 1296.99 m (V C-N), 1104.09 (V C-S), 678.42 {out-of-plane stretch C-H(Ar)}. ...
Embodiment 2
[0030] A kind of sulfide heavy metal collector N 1 ,N 2 ,N 4 ,N 5 -Tetrakis(2-mercaptoethyl) 1,2,4,5-pyromelliticamide, the preparation method of which is as follows:
[0031] At 5°C, in a 150mL three-neck flask, dissolve mercaptoethylamine hydrochloride to pyromellitic anhydride at a molar ratio of 4.8, namely 5.46 g of mercaptoethylamine hydrochloride and 2.18 g of pyromellitic anhydride in 75 mL of dimethylformaldehyde In the amide, react under stirring for 6 hours to obtain the heavy metal chelating collector N 1 ,N 2 ,N4 ,N 5 -Tetrakis(2-mercaptoethyl) 1,2,4,5-pyromellitic tetracarboxamide (TMBTC for short), the reaction yield is 51.62%.
Embodiment 3
[0033] A kind of sulfide heavy metal collector N 1 ,N 2 ,N 4 ,N 5 -Tetrakis(2-mercaptoethyl) 1,2,4,5-pyromelliticamide, the preparation method of which is as follows:
[0034] At -5°C, in a 150mL three-necked flask, dissolve mercaptoethylamine hydrochloride to pyromellitic anhydride at a molar ratio of 5.2, that is, 5.91 g of mercaptoethylamine hydrochloride and 2.18 g of pyromellitic anhydride are dissolved in 75 mL of dimethyl In formamide, react under stirring for 8 hours to obtain the heavy metal chelate collector N 1 ,N 2 ,N 4 ,N 5 -Tetrakis(2-mercaptoethyl) 1,2,4,5-pyromellitic tetracarboxamide (TMBTC for short), the reaction yield is 76.17%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com