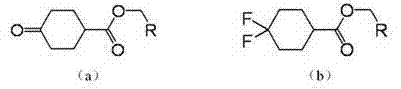Method for producing 4,4-difluoro cyclohexyl formic ether by using counter cyclohexanone formic ether through fluorination
A technology of difluorocyclohexyl carboxylate and cyclohexanone carboxylate, applied in chemical instruments and methods, preparation of carboxylic acid esters, preparation of organic compounds, etc. Low efficiency and other problems, to achieve the effect of large-scale production, no risk of explosion, and less reaction by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This embodiment adopts ethyl cyclohexanone formate to produce ethyl 4,4-difluorocyclohexylcarboxylate through fluorination, and its production steps are:
[0034] (1) Weigh 42.5g (0.25mol) of ethyl p-cyclohexanone formate and put it into the autoclave, put in 5g (0.25mol) of hydrogen fluoride, and feed 27g of sulfur tetrafluoride into the autoclave from the sulfur tetrafluoride cylinder (0.25mol), start stirring, control the temperature at 10°C, and react for 12 hours;
[0035] (2) After the reaction is over, open the outlet valve of the kettle to vent the remaining gas to the pre-prepared 500 ml 10% Na 2 CO 3 In the solution, the gas is absorbed by the lye;
[0036] (3) After the gas is released, open the autoclave, slowly pour the reaction liquid into 200ml of ice water (the ice water is a mixture of ice and water, the temperature is 0°C), stir at the same time, let it stand for stratification, and separate the oil layer;
[0037] (4) NaHCO for oil layer 3 The aqu...
Embodiment 2
[0040] The basic implementation steps are the same as in Example 1, except that the amount of hydrogen fluoride is changed to 2.5 g (0.125 mol), 10 g (0.5 mol), 30 g (1.5 mol), 50 g (2.5 mol), respectively, and the purity of the crude product is 82.5 %, 86.2%, 90.4%, 88.5%, and after distillation, the weights of light yellow liquid with a content of more than 99% are: 30.5g, 33.5g, 35.2g, 34.9g.
Embodiment 3
[0042] The basic implementation steps are the same as in Example 1, the amount of sulfur tetrafluoride is changed to 54g (0.5mol), 81g (0.75mol), 108g (1.0mol) respectively, and the purity of the crude product is 88%, 86.2%, 81.5% respectively After distillation, the weights of light yellow liquids with a content of more than 99% are: 34.5 g, 33.5 g, and 29.5 g, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com