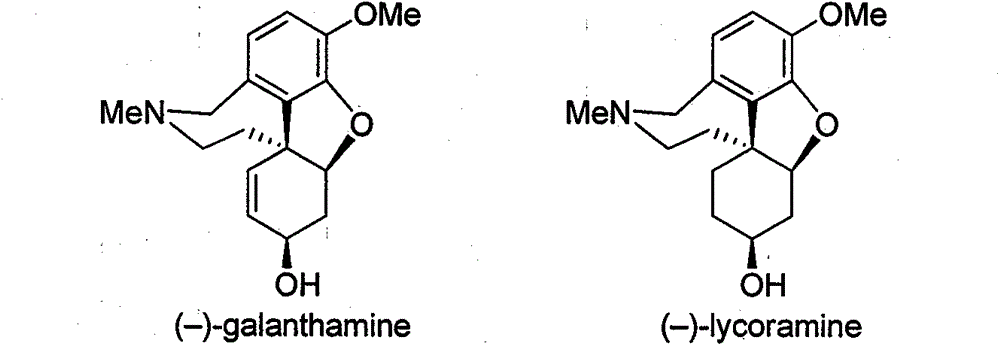
Asymmetric synthesis method for galanthamine and lycoramine
A technology of galantamine and synthetic method, which is applied in the field of asymmetric synthesis of natural products, and can solve the problems of lack of large-scale production, high-efficiency asymmetric catalytic construction of chiral centers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
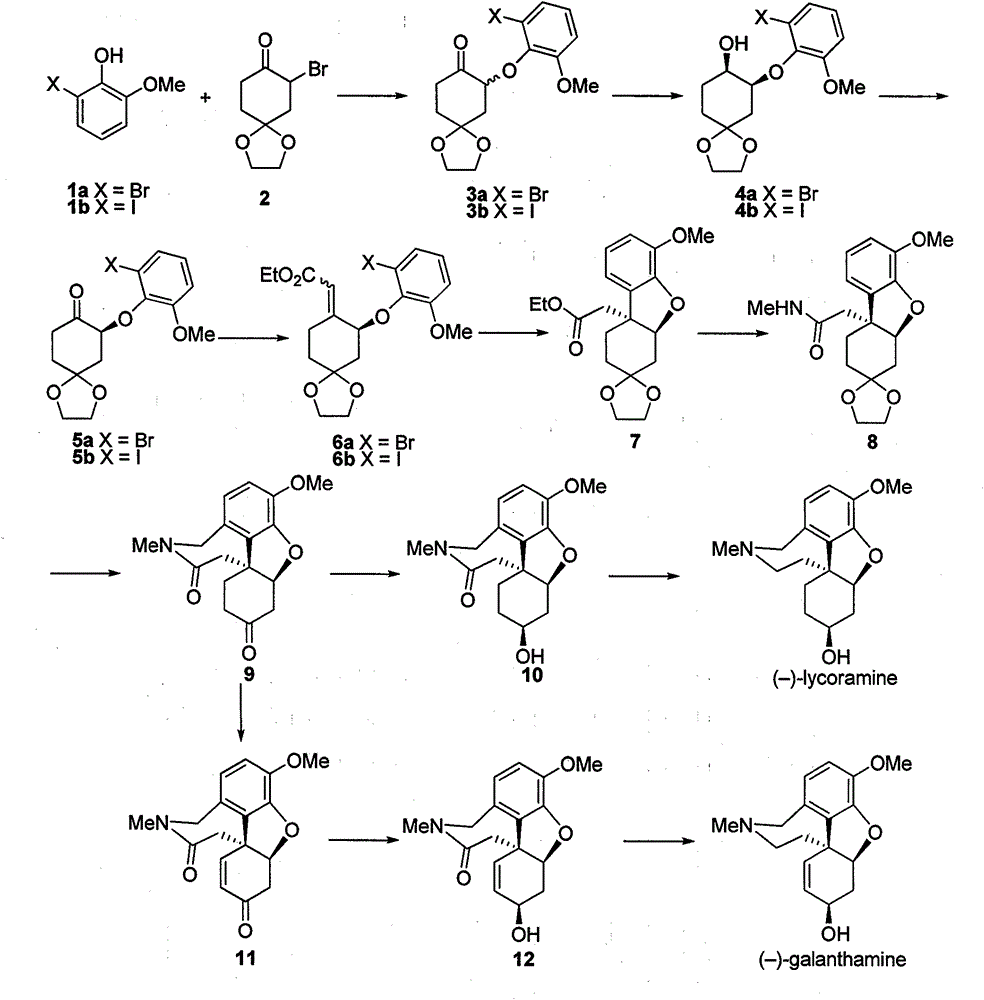
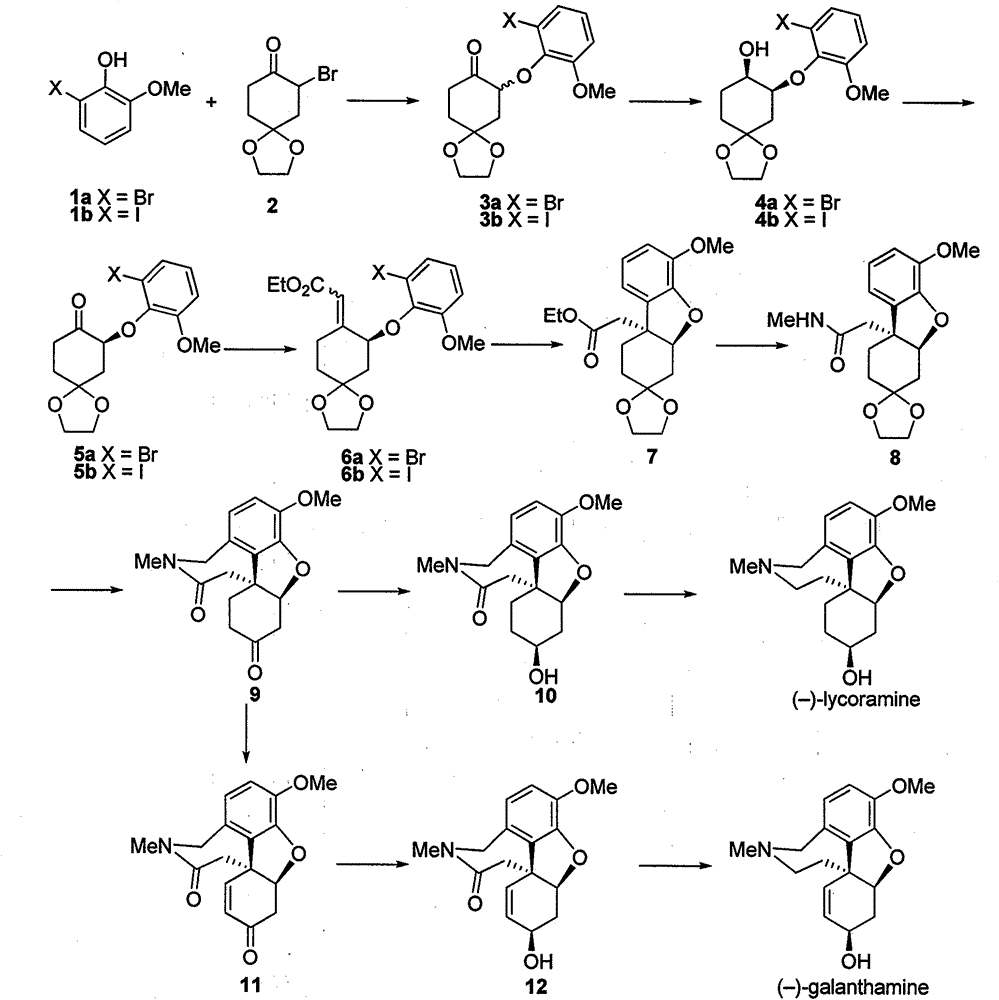
[0025] Preparation of compound 3a
[0026] Add compound 1a (15.1g, 74.3mmol), compound 2 (30.0g, 89.2mmol) and anhydrous potassium carbonate (10.3g, 74.3mmol) successively to a dry 250mL two-necked flask with a reflux condenser, add 150mL DMF Dissolve under electromagnetic stirring, and heat the oil bath to 80°C for 8 hours. The reaction was stopped after the disappearance of compound 2 was detected by TLC, and the system was cooled to room temperature. Suction filtration through celite, and the filtrate was concentrated in vacuo to obtain an oily liquid. Add 100 mL of ethyl acetate to dissolve the oil, and wash with water and saturated brine successively. The organic phase was dried with anhydrous sodium sulfate, and the solvent was removed. The resulting oily liquid was subjected to silica gel column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain 18.6 g of a white solid, with a yield of 70%.
[0027] Mp: 88-90°C; 1 HNMR (400MHz, CDCl 3 )δ7.13(d, J=8.0H...
Embodiment 2
[0029] Preparation of compound 3b
[0030] Using the same operation as in Example 1, 22.5 g of a white solid was obtained, with a yield of 75%.
[0031] Mp: 77-78°C; 1 HNMR (400MHz, CDCl 3 )δ7.34(dd, J=7.9, 1.0Hz, 1H), 6.85(d, J=8.1 Hz, 1H), 6.75(t, J=8.0Hz, 1H), 5.05(dd, J=10.5, 8.1 Hz), 4.01-3.96(m, 4H), 3.76(s, 3H), 2.70-2.59(m, 1H), 2.53(dt, J=14.1, 4.3Hz, 1H), 2.43-2.36(m, 2H) , 2.04 (dd, J=11.1, 4.6Hz, 2H). 13 CNMR (100MHz, CDCl 3 )δ205.2, 151.8, 146.6, 131.1, 125.6, 113.0, 107.6, 92.7, 81.2, 64.8, 64.7, 56.1, 42.1, 35.9, 34.6. HRMS (ESI, [M+Na] + ): calculated: 427.0013, found: 427.0010.
Embodiment 3
[0033] Preparation of compound 4a
[0034] Weigh out RuCl in the glove box 2 -(R)-SDP / (S,S)-DPEN (11.0mg, 0.01mmol) was placed in the inner tube of a 100mL reactor, sealed with a sealant, taken out, placed in the reactor, and injected into 8.0mL i PrOH, after peeling off the sealant, quickly replace the hydrogen and keep the hydrogen pressure at 25atm. Stir at room temperature for about 5 minutes to fully dissolve the catalyst, release hydrogen under reduced pressure, and then add dissolved compound 3 (3.57g, 10mmol) i PrOH solution 22mL O t Bu's i PrOH solution (0.2 mmol / mL, 5.0 mL. 1.0 mmol). After the addition is complete, the hydrogen is replaced again and the initial hydrogen pressure is 30 atm, and the reaction is carried out under electromagnetic stirring at room temperature for 3 hours until the hydrogen pressure does not drop. The remaining hydrogen was released under reduced pressure, the inner tube was taken out, the solvent was spin-dried, water was added, ext...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com