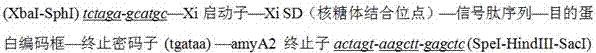Construction method for streptomycete expression plasmids and production method for keratinase
A technology of expression plasmid and construction method, which is applied in the field of construction of Streptomyces expression plasmid, and can solve the problems of lack of advantages in induced expression, inability of expression amount to meet industrial production requirements, and low expression level.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1, keratinase gene ( sfks ) of the separation
[0036] According to Streptomyces flexneri k-11 published online ( Streptomyces fradiae var. k-11) Keratinase gene sfp 2 (EMBL accession number AJ784940) sequence, designed primers
[0037] sfks PF: 5'-AACATATGCGCTTCACCCCCCG-3' (SEQ ID NO: 3)
[0038] sfks PR: 5'-GGGATCCGTCAGATGATGCTGACG-3' (SEQ ID NO: 4).
[0039] Amplified by this primer sfks Genes, including keratinase maturation enzyme gene (encoding 191aa), an N-terminal leader peptide (encoding 78aa) and Streptomyces exocrine signal peptide (encoding 38aa).
[0040] The reaction system is: 10×KOD-plus buffer 5μl, dNTP mix (2.5mmol / L) 5μl, MgSO 4 (25μM) 1.5μl, 50%DMSO 3μl, template DNA 1μl, Primer R (20μM) 1μl, Primer F (20μM) 1μl, KOD-plus (1U / μl) 1μl, ddH 2 O 31.5 μl. The reaction conditions were: denaturation at 95°C for 5min, 30s at 95°C, 30s at 65°C, extension at 72°C for 1min30s, a total of 30 cycles, and 5min at 72°C. Th...
Embodiment 2
[0041] Embodiment 2, expression plasmid construction
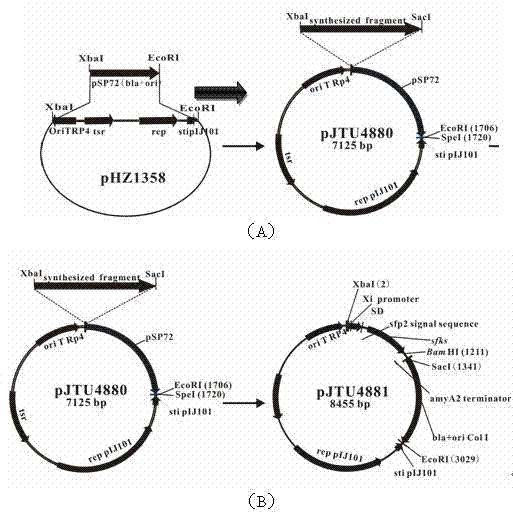
[0042] In the present invention, pSP72 is double-digested with XbaI and EcoRI, and a 1700bp fragment is recovered. Similarly, pHZ1358 is digested with XbaI and EcoRI to obtain a 5424bp fragment. The two fragments are connected by PCR to construct plasmid pJTU4880, and then the cloned sfks The gene is inserted into the synthetic Xi promoter-SD (ribosome binding site)-amyA2 terminator fragment to construct the expression box structure, and the expression box structure schematic diagram is as follows figure 2 As shown, its corresponding expression box gene sequence is shown in SEQ ID NO:5:
[0043] tctagagcatgcgcccaccggcgatcaggtcgtcgacgagcgcggagacggtggcccgggtgagcccggtgacggcggcaactcccgcgcgggagagccgatctgtgctgtttgccacggtatgcagcaccagcgcgagattatgggctcgcacgctcgactgtcggacgggggcactggaacgagaagtcaggcgagccgtcacgcccttgacaatgccacatcctgagcaaataattcaaccactaaacaaatcaaccgcgtttcccggaggatgcagtgaacctcaagcgcttcaccccccgcggcggactcgcgagagg...
Embodiment 3
[0045] Embodiment 3, heterologous expression of keratinase
[0046] Transformation of the target plasmid with oriT into Escherichia coli ET12567 (pUZ8002) (Tao Weixin, Wu Jing, Deng Zixin, Tao Meifeng. Cloning of bldA in Streptomyces avermitilis NLLL8165 and its effect on morphological differentiation and abamectin synthesis. Microbial After the competent cells in Acta Sinica (2007) 47 (1): 34-38), the transformed cells were cultured overnight, and the culture was inoculated in fresh LB with an inoculum of 1:20, and cultured at 37°C for 3-4 hours; collected sky blue Streptomyces spores were washed once with sterile water, centrifuged at 4500rpm for 3min, and the supernatant was washed dry, then washed once with 0.05 mol / L TES (pH8.0), heat-shocked at 50°C for 10min, cooled in water and then added with 750μL spore germination solution , incubate at 37°C for 2 hours, centrifuge at 4500rpm for 10min, wash the pellet twice with LB to remove antibiotics, and finally suspend i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com