Lansoprazole lyophilized powder injection preparation and its preparing method
A technology of lansoprazole and freeze-dried powder injection, which is applied in the field of lansoprazole freeze-dried powder injection and its preparation, can solve problems such as unstable quality of lansoprazole freeze-dried powder preparation, and achieve appearance properties Good, stable appearance, stable content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
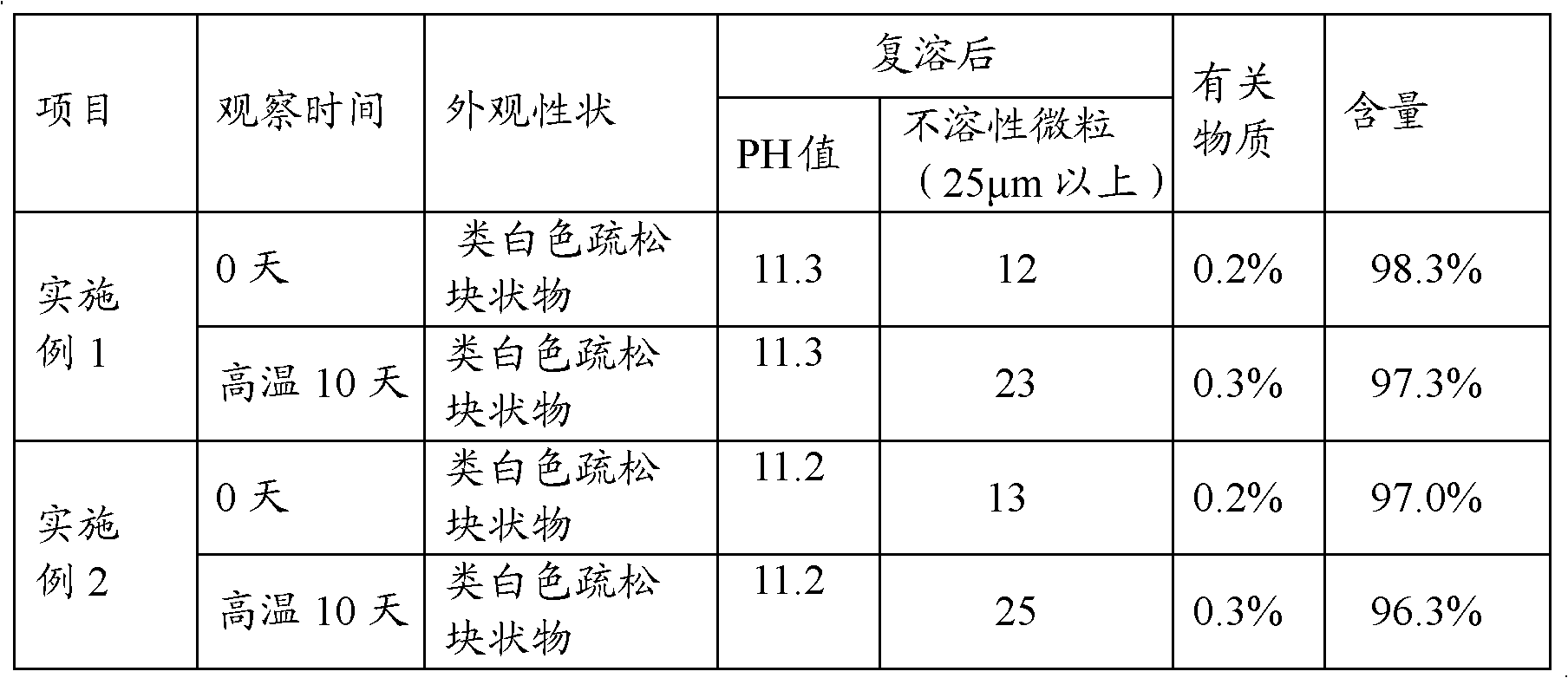
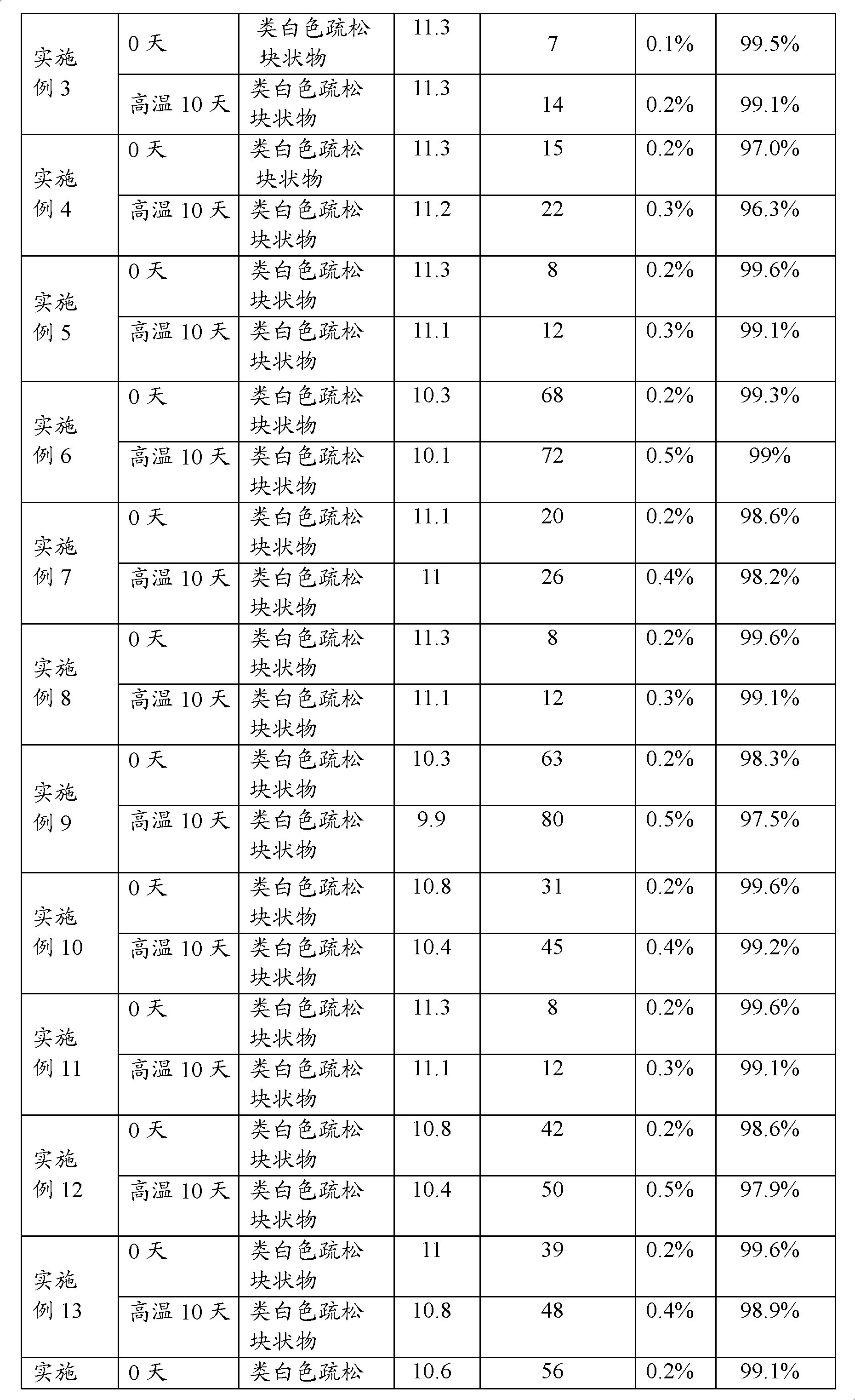
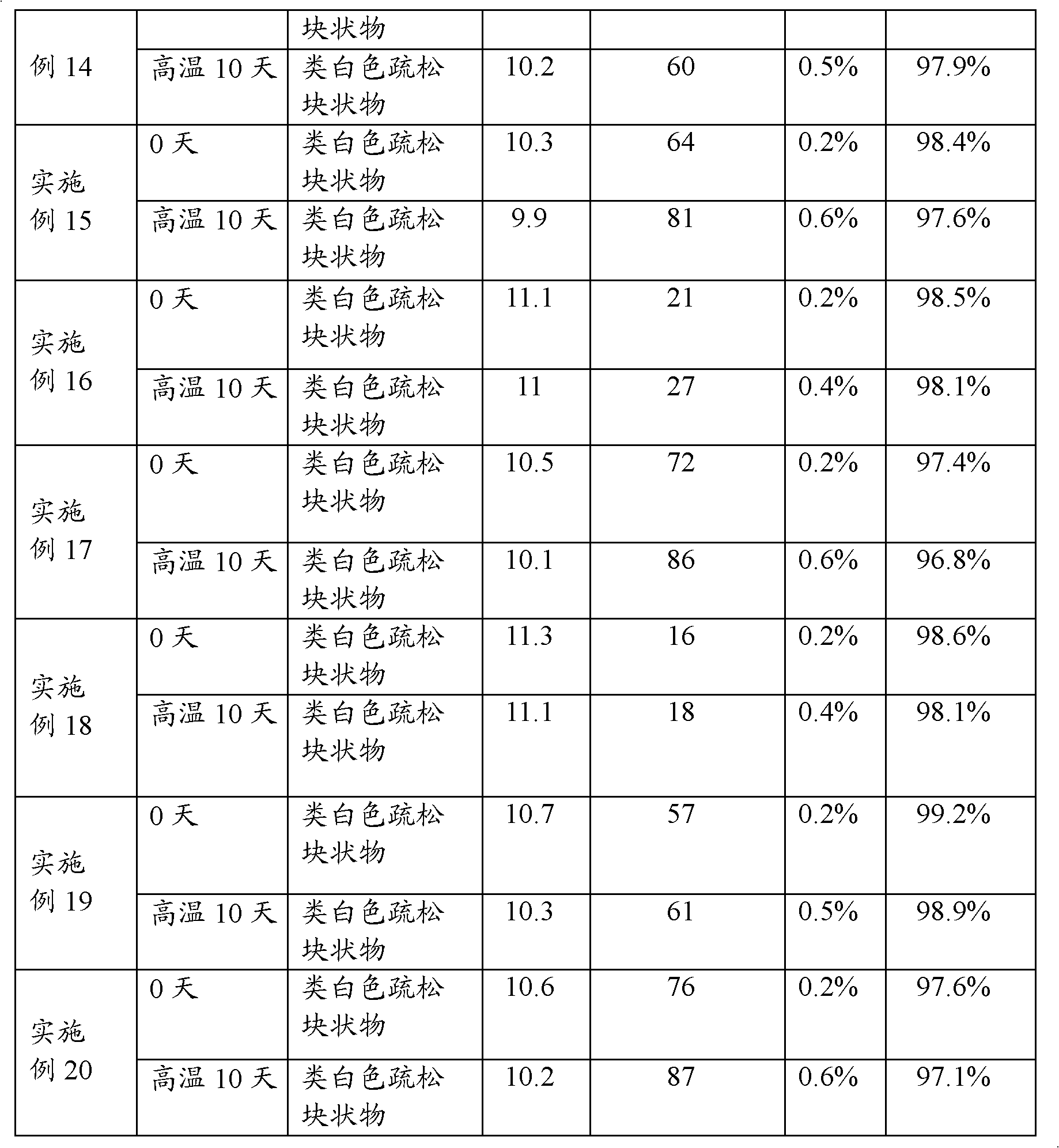
Examples
Embodiment 1
[0032] (1) Add 3200ml of water for injection at room temperature to 60°C into the preparation tank, add 8g of meglumine, 0.05g of calcium sodium edetate, 4.9g of sodium carbonate, and 210g of mannitol, stir to dissolve, and then add 29g of lansoprazole , and stir until completely dissolved.
[0033] (2) Adjust the pH value of the solution to 11.4-12.0 with 50 ml of 1 mol / L sodium hydroxide aqueous solution. Add 5g of medicinal activated carbon to the solution to absorb impurities, add water for injection to 4000ml, stir at room temperature for 30 minutes, filter and decarbonize, then sterilize and filter with a 0.22μm microporous filter membrane, and fill it evenly in a sterile environment There are about 1000 bottles in glass bottles.
[0034] (3) Put the medicinal glass bottle into a freeze-drying box, lower the temperature to -45°C, and gradually increase the temperature in a vacuum state to implement freezing and drying. During the freezing process, the following tempera...
Embodiment 2
[0037] (1) Add 3200ml of water for injection at room temperature to 60°C into the preparation tank, add 8g of meglumine, 0.02g of calcium sodium edetate, 5.3g of sodium carbonate, and 215g of mannitol, stir to dissolve, and then add 30g of lansoprazole , and stir until completely dissolved.
[0038] (2) Adjust the pH value of the solution to 11.4-12.0 with 50 ml of 1 mol / L sodium hydroxide aqueous solution. Add 5g of medicinal activated carbon to the solution to absorb impurities, add water for injection to 4000ml, stir at room temperature for 30 minutes, filter and decarbonize, then sterilize and filter with a 0.22μm microporous filter membrane, and fill it evenly in a sterile environment Glass bottle of 1000 bottles.
[0039] (3) Put the medicinal glass bottle into a freeze-drying box, lower the temperature to -45°C, and gradually increase the temperature in a vacuum state to implement freezing and drying. During the freezing process, the following temperature program is use...
Embodiment 3
[0042] (1) Add 3200ml of water for injection at normal temperature to 60°C in the preparation tank, add 8g of meglumine, 0.2g of polyethylene glycol, 4.9g of sodium carbonate, 100g of oligodextran, and 100g of mannitol, stir to dissolve, and then Add Lansoprazole 30g, stir until all dissolve.
[0043] (2) Adjust the pH value of the solution to 9.5-10.5 with 45 ml of 1 mol / L sodium hydroxide aqueous solution. Add 5g of medicinal activated carbon to the solution to absorb impurities, add water for injection to 4000ml, stir at room temperature for 30 minutes, filter and decarbonize, then sterilize and filter with a 0.22μm microporous filter membrane, and fill it evenly in a sterile environment There are about 1000 bottles in glass bottles.
[0044] (3) Put the medicinal glass bottle into a freeze-drying box, lower the temperature to -45°C, and gradually increase the temperature in a vacuum state to implement freezing and drying. During the freezing process, the following temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com