Trimetazidine sustained release capsule and preparation method thereof
A slow-release capsule and trimetazidine technology, applied in the field of medicine, can solve the problems of long coating cycle, uneconomical, large coating amount, etc., and achieve the effects of reducing drug resistance, eliminating peak concentration, and rapidly absorbing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
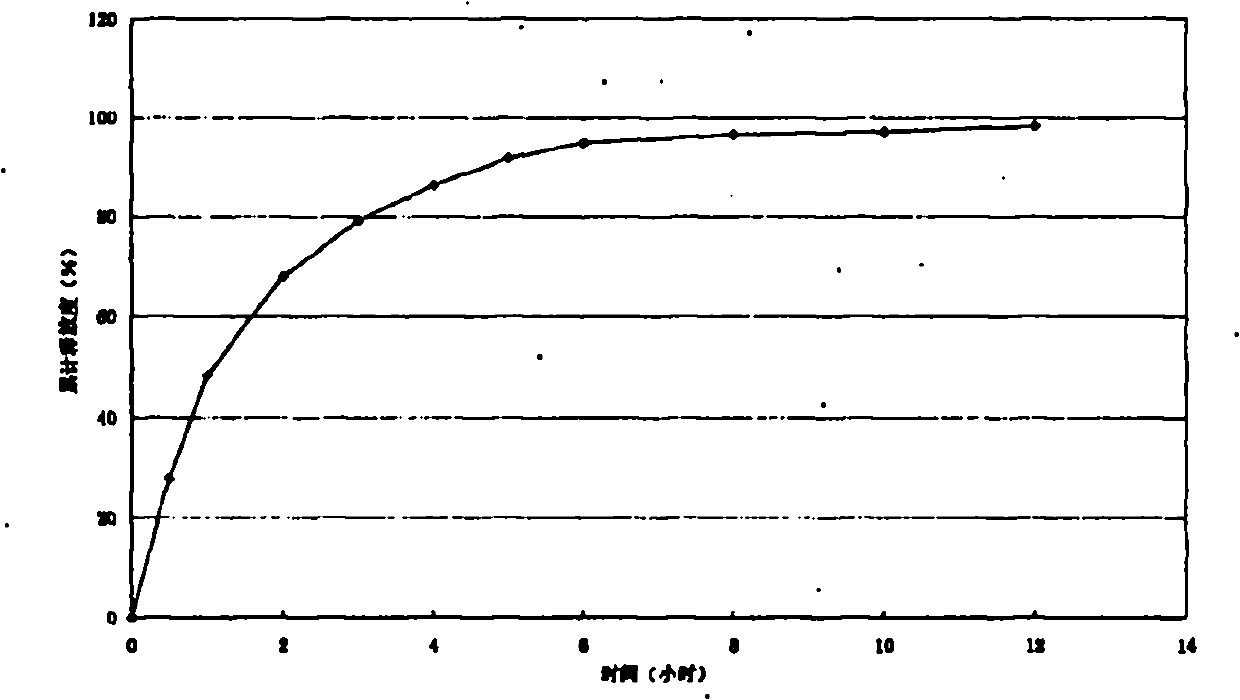
Embodiment 1
[0029] Trimetazidine hydrochloride sustained-release capsules contain the following components:
[0030] Trimetazidine Hydrochloride 35g
[0031] Hydroxypropylmethylcellulose (HPMC) 10g
[0032] Blank Base Pills 200g
[0033] Eudragit RS 100 20g
[0034] Triethyl citrate 1.2g
[0036] ------------------------------
[0037] Total 280.2g
[0038] Preparation Process:
[0039] Preparation of drug-containing pellets: Weigh the hydroxypropyl methylcellulose required in the prescription and dissolve it in pure water to make HPMC solution, then add the prescribed amount of trimetazidine hydrochloride, and mix it in a high-shear mixing emulsifier Uniform suspension; put the prescribed amount of blank pellets into the coating pan, adjust the speed of the coating pan, preheat, adjust the blowing temperature, so that the temperature of the pellet bed reaches the required level, spray the above suspension evenly into the coating pan In the blank pellets;...
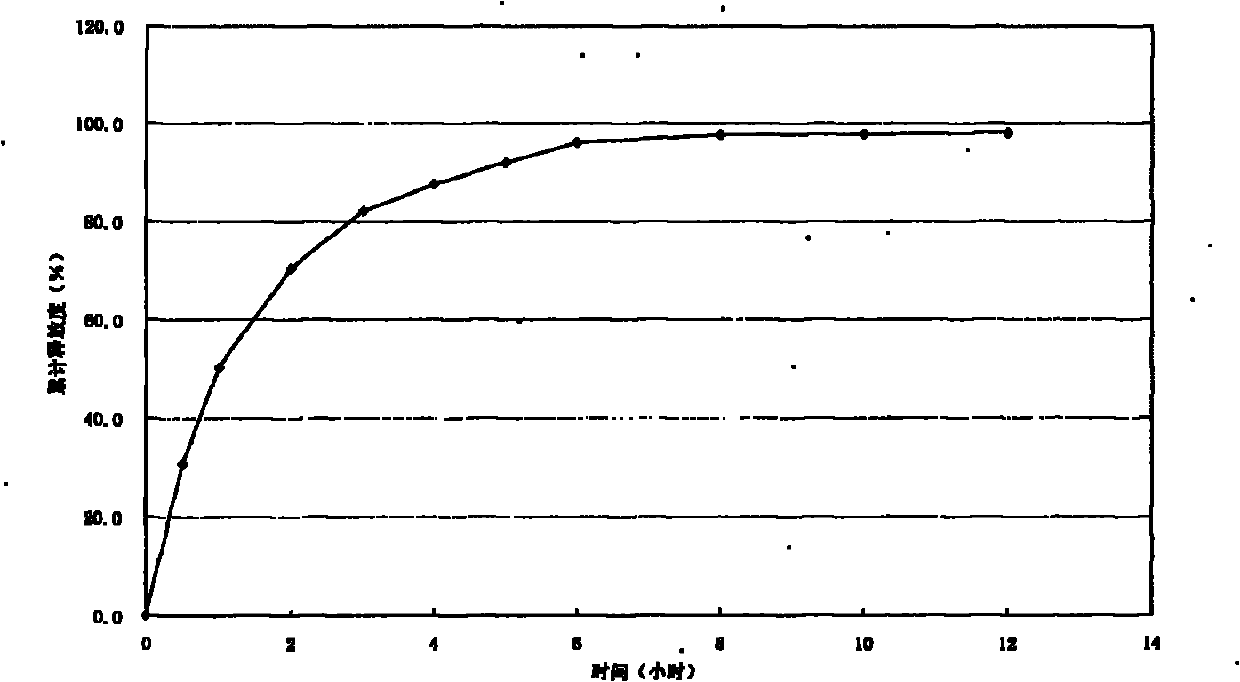
Embodiment 2
[0043] Trimetazidine hydrochloride sustained-release capsules contain the following components:
[0044] Trimetazidine Hydrochloride 35g
[0045] Hydroxypropylmethylcellulose (HPMC) 9g
[0046] Blank Base Pills 180g
[0047] Eudragit RS 100 20g
[0048] Eudragit S 100 10g
[0049] Triethyl citrate 2.7g
[0051] --------------------------------
[0052] Total 283.7g
[0053] Preparation Process:
[0054] Preparation of drug-containing pellets: Weigh part of the hydroxypropyl methylcellulose required in the prescription and dissolve it in pure water to make an HPMC solution, then add the prescribed amount of trimetazidine hydrochloride, and mix it in a high-shear mixing emulsifier. into a uniform suspension; put the prescribed amount of blank base pellets into the fluidized bed, adjust the blowing temperature, adjust the air volume, preheat, so that the temperature of the pellet bed reaches the required level, and spray the suspension into the bla...
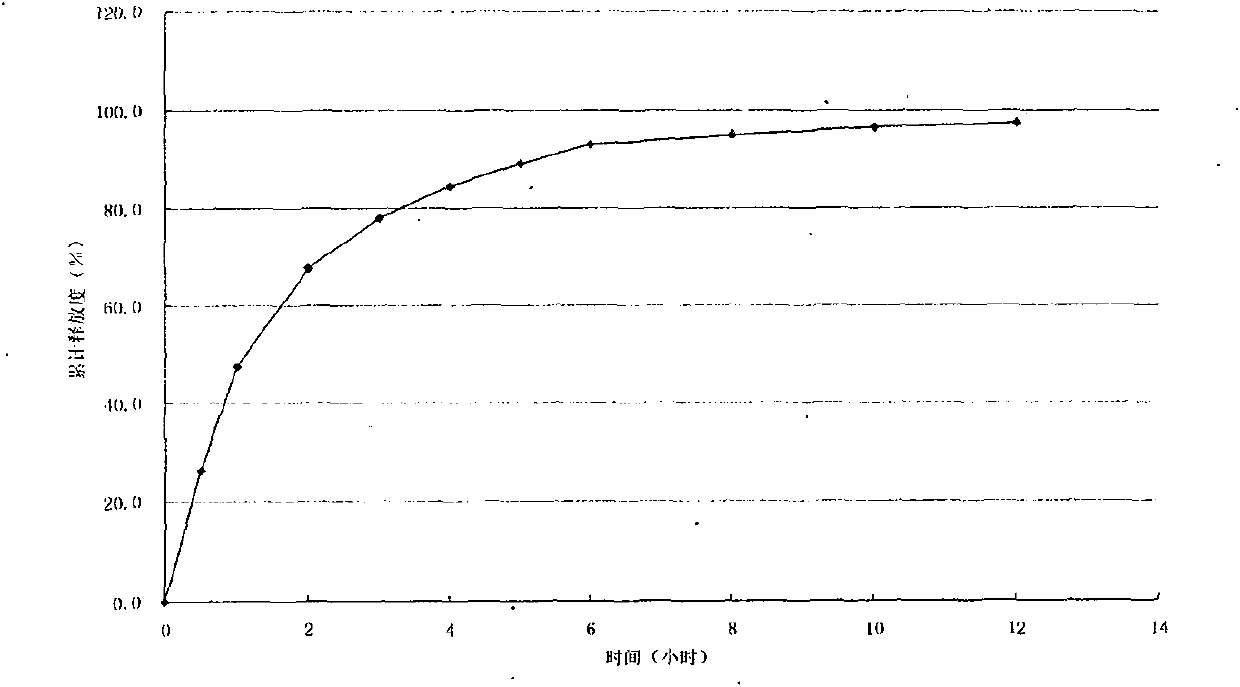
Embodiment 3
[0058] Trimetazidine hydrochloride sustained-release capsules contain the following components:
[0059] Trimetazidine Hydrochloride 35g
[0060] Hydroxypropylmethylcellulose (HPMC) 10g
[0061] Blank Base Pills 180g
[0062] Eudragit RS30D 25g
[0063] Eudragit L30D-55 5g
[0064] Triethyl citrate 2.2g
[0066] --------------------------
[0067] Total 279.2g
[0068] Preparation Process:
[0069] Preparation of drug-containing pellets: Weigh the hydroxypropyl methylcellulose required in the prescription and dissolve it in pure water to make HPMC solution, then add the prescribed amount of trimetazidine hydrochloride, and mix it in a high-shear mixing emulsifier Uniform suspension; put the prescribed amount of blank base pills into the fluidized bed, adjust the blowing temperature, adjust the air volume, preheat to make the temperature of the pellet bed reach the required level, and spray the above suspension into the blank pellets evenly; T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com