Stable composite fat-soluble vitamin composition and preparation method thereof
A technology of vitamins and compound lipids, which is applied in the direction of drug combinations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as unsupplied, unstable freeze-dried preparations, and unstable physical and chemical properties. Achieve the effects of improved stability, excellent stability, and low manufacturing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
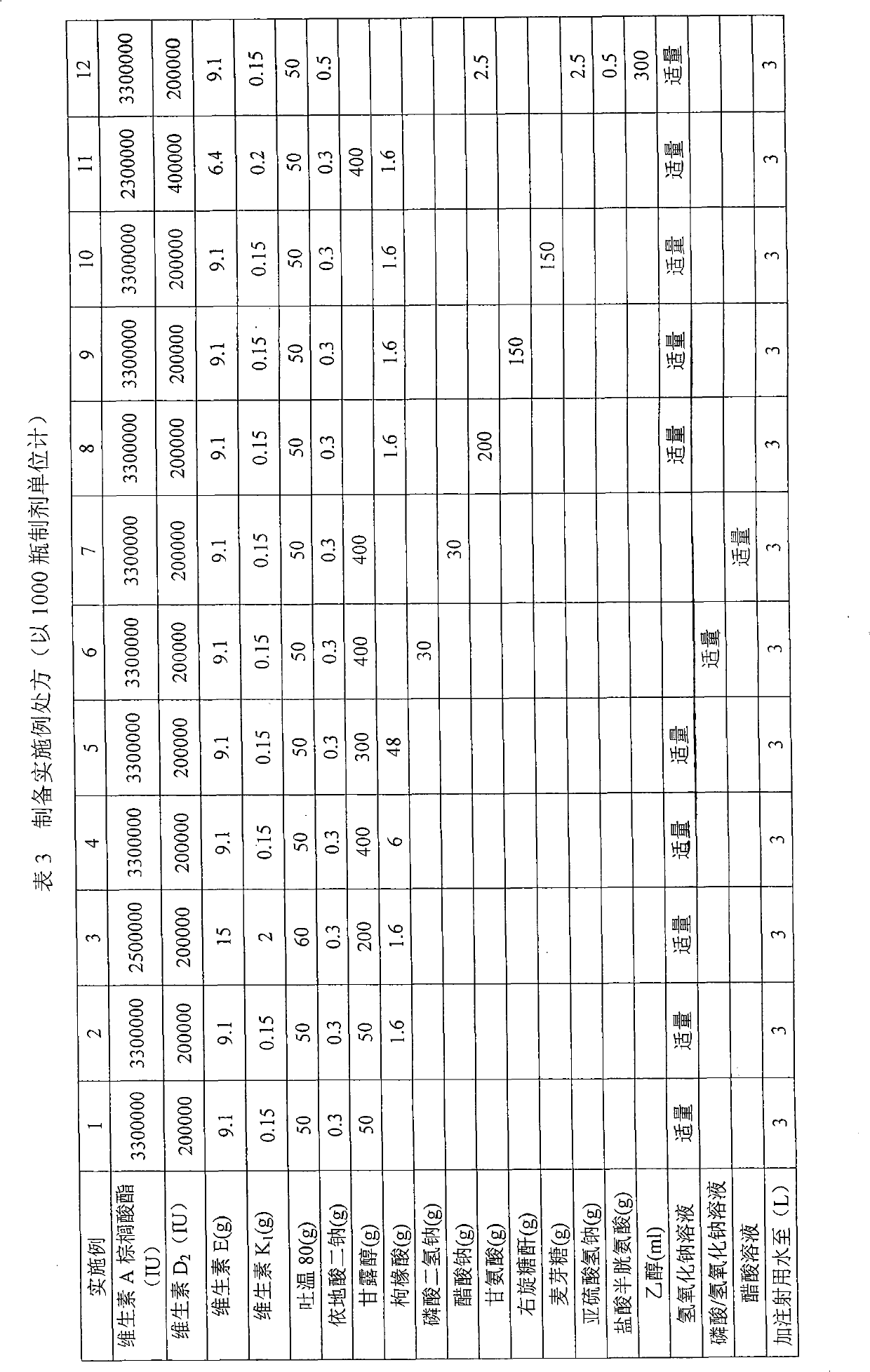
Embodiment 1
[0092] Preparation Process:
[0093] (1) Weigh vitamin A palmitate, vitamin E, and vitamin K in a light-proof operating room 1 , Vitamin D 2 , added to polysorbate 80, charged with nitrogen, stirred to dissolve, added with water for injection, stirred, and placed for later use.
[0094] (2) Take edetate disodium and mannitol, add water for injection and stir to dissolve.
[0095] (3) Mix the above (1) and (2) evenly, adjust the pH value with sodium hydroxide solution, add 0.05% activated carbon, stir for 30 minutes, filter, replenish the water for injection to the full amount, measure the intermediate content, pH value, fine Filter and fill.
[0096] (4) The sample is freeze-dried to obtain.
Embodiment 2
[0098] Preparation Process:
[0099] (1) Weigh vitamin A palmitate, vitamin E, and vitamin K in a light-proof operating room 1 , Vitamin D 2 , added to polysorbate 80, charged with nitrogen, stirred to dissolve, added with water for injection, stirred, and placed for later use.
[0100] (2) Get disodium edetate, mannitol, and citrate, add water for injection and stir to dissolve, and adjust the pH value with sodium hydroxide solution.
[0101] (3) Mix the above (1) and (2) evenly, add 0.05% activated carbon, stir for 30 minutes, filter, add water for injection to the full amount, measure the intermediate content and pH value, fine filter, and fill.
[0102] (4) The sample is freeze-dried to obtain.
Embodiment 3
[0104] Preparation process: as in Example 2, it is prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com