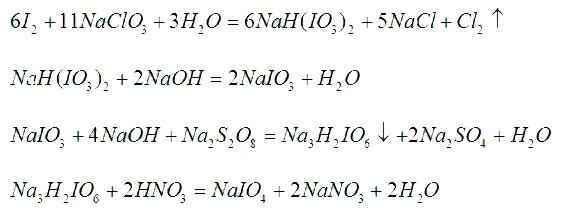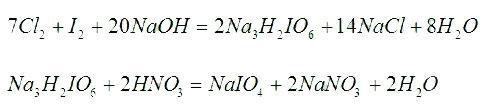Method for producing sodium periodate
A sodium periodate and production method technology, applied in the direction of iodine oxygen compounds, etc., can solve problems such as low safety, large consumption of raw materials, difficult operation control, etc., to achieve environmental protection, high production safety, and small consumption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: a kind of production method of sodium periodate comprises successively the preparation step of dihydrotrisodium periodate, the preparation step of sodium periodate, the crystallization separation step, centrifugal dehydration step and drying step, wherein,
[0018] (1) The preparation steps of trihydrogen trisodium periodate are:
[0019] a. The preparation step of sodium hydrogen iodate: put into the water of 700~800L in the reactor, be 11:6:3 to weigh sodium chlorate and iodine according to the mol ratio of sodium chlorate, iodine and water, put it into Put it into the reaction kettle, add an appropriate amount of concentrated nitric acid to adjust the pH value of the solution to ≤1, heat the reaction kettle for 0.5 hours, then open the steam valve of the reaction kettle and the chlorine-removing valve to chase chlorine for 150 minutes, expel the chlorine gas and absorb it with lye until the inside of the reaction kettle turns red. disappear, the solutio...
Embodiment 2
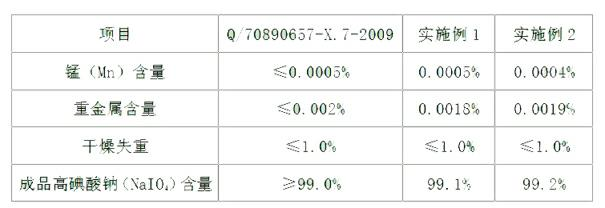
[0030] Embodiment 2: a kind of production method of sodium periodate comprises the preparation step of dihydrotrisodium periodate, the preparation step of sodium periodate, the crystallization separation step, centrifugal dehydration step and drying step successively, wherein,
[0031] (1) The preparation steps of trihydrogen trisodium periodate are:
[0032]a. The preparation step of sodium hydrogen iodate: put into the water of 700~800L in the reactor, be 11:6:3 to weigh sodium chlorate and iodine according to the mol ratio of sodium chlorate, iodine and water, put it into Put it into the reaction kettle, add an appropriate amount of concentrated nitric acid to adjust the pH value of the solution to ≤1, heat the reaction kettle for 1 hour, then open the steam valve of the reaction kettle and the chlorine-removing valve to chase chlorine for 180 minutes, expel the chlorine gas and absorb it with lye until the inside of the reaction kettle is red disappear, the solution is cle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com