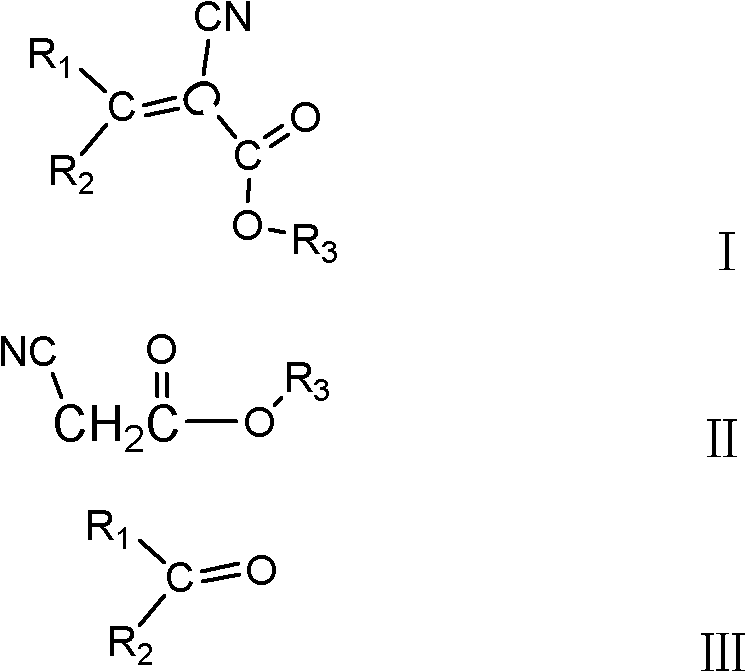
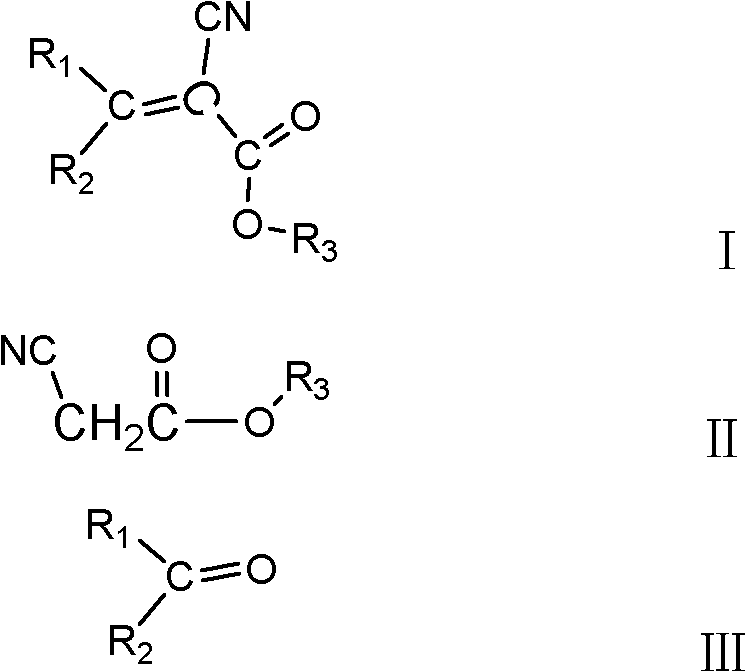
Method for producing 2-cyanoacrylate
A technology of cyanoacrylate and cyanoacetate, which is applied in the field of 2-cyanoacrylate production, can solve the problems of difficult production control, shortened reaction time, increased production cost, etc., so as to avoid catalyst deactivation, Strong operability and waste reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] In a four-necked flask with stirring and a thermometer, put 198g (1mol) of 2-ethylhexyl cyanoacetate, 546.66g (3mol) of benzophenone, and 161.1g of catalyst solution (comprising 80.55g of acetic acid, 38.54g of ammonium acetate and 42.01g sodium bicarbonate), 200g heptane, install the water separator and condenser, gradually raise the reaction temperature to reflux at 105°C, add 10g catalyst solution every 1h, and simultaneously separate the lower aqueous solution of the condensate, heptane Flow back into the four-necked flask. Sampling is performed to detect that the content of 2-ethylhexyl cyanoacetate is less than 3 wt%, and the reaction is terminated, and the content of amide impurities is less than 0.4 wt%. Heptane was distilled off under normal pressure, acetic acid was distilled off under reduced pressure until the content of acetic acid was less than 0.5wt% as the end point, 20wt% ammonium bicarbonate was added to neutralize to pH=7, and the upper oil phase was ...
Embodiment 2
[0034] In a four-necked flask with stirring and a thermometer, drop 396g (2mol) cyanoacetate-2-ethylhexyl, 728.88g (4mol) benzophenone, 530.16g catalyst solution (463.89g acetic acid, 30.83g ammonium acetate, 35.44g of acetamide), 200g of butyl acetate, install a water separator and a condenser, gradually raise the reaction temperature to reflux at 110°C, add 20g of catalyst solution every 1h, and simultaneously separate the lower aqueous solution of the condensate, and the heptane flow Return to the four-necked flask. Sampling is performed to detect that the content of 2-ethylhexyl cyanoacetate is less than 2%, the reaction is terminated, and the content of amide impurities is less than 0.5 wt%. Distill butyl acetate under normal pressure, distill acetic acid under reduced pressure until the content of acetic acid is less than 0.5wt% as the end point, add 25wt% sodium carbonate to neutralize to pH = 7, and separate the upper oil phase. The oily phase was distilled under reduce...
Embodiment 3
[0036] Utilize cyanoacetic acid and isooctyl alcohol to obtain -2-ethylhexyl cyanoacetate under the catalysis of sulfuric acid, in the four-necked flask with stirring, thermometer, drop 594g (3mol) -2-ethylhexyl cyanoacetate, 546.66 g (3mol) benzophenone, 386.72g catalyst solution (comprising 290.04g acetic acid, 25.22g cyanoacetamide and 71.46g malonamide), 200g dimethyl carbonate, install water trap, condenser, the reaction temperature Gradually raise to reflux at 80°C, add 10g of catalyst solution every 1h, and at the same time separate the lower aqueous solution of the condensate, and return the heptane to the four-necked flask. Sampling is performed to detect that the content of 2-ethylhexyl cyanoacetate is less than 3 wt%, and the reaction is terminated, and the content of amide impurities is less than 0.4 wt%. Dimethyl carbonate was distilled off under normal pressure, acetic acid was distilled off under reduced pressure until the content of acetic acid was less than 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com