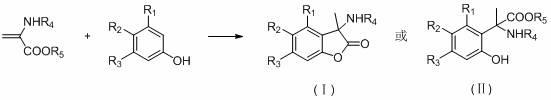
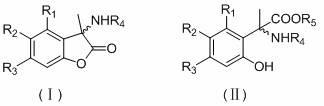
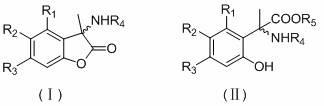
Alpha-aryl alanine compounds and preparation method thereof
A technology for aryl alanine and compounds, applied in the fields of medicinal chemistry and anti-infection therapeutics, can solve the problems of low reaction yield, long steps, high cost, etc., and achieve the effects of high yield, mild reaction conditions and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 3-methyl, 3-(N-acetyl)-5-allyloxy-6-methoxybenzofuran lactone;
[0019] To a solution of methyl 2-acetamidoacrylate (429.4 mg, 3 mmol) in 10 mL of dry THF was added 1.58 mL of BF at room temperature 3 ·Et 2 O (6mmol), after stirring for 0.5 hours, add 5 milliliters of 3-methoxy-4-allyloxyphenol (434 mg, 2.4 mmol) in anhydrous tetrahydrofuran solution, continue to react at room temperature for 48 hours, and distill under reduced pressure to remove Remove the solvent, dissolve the residue with an appropriate amount of ethyl acetate, add saturated sodium bicarbonate solution, extract and separate layers, extract the aqueous phase with ethyl acetate three times, combine the organic phases and wash with saturated brine, and wash the obtained ethyl acetate solution with anhydrous sulfuric acid Sodium drying, filtering to remove the desiccant, and the residue obtained after vacuum distillation was separated by silica gel column chromatography (V petroleum ether / V et...
Embodiment 2
[0020] Example 2 3-methyl, 3-(N-acetyl)-5-allyloxybenzofuran lactone:
[0021] To a solution of methyl 2-acetamidoacrylate (72 mg, 0.5 mmol) in 5 mL of dry THF was added 0.26 mL of BF at room temperature 3 ·Et 2 O (0.99 mmol), after stirring for 0.5 hours, add 2 milliliters of 4-allyloxyphenol (60 mg, 0.4 mmol) in anhydrous tetrahydrofuran solution, continue the reaction at room temperature for 48 hours, remove the solvent by distillation under reduced pressure, and the remaining The mixture was dissolved with an appropriate amount of ethyl acetate, then saturated sodium bicarbonate solution was added, the layers were extracted, the aqueous phase was extracted three times with ethyl acetate, the organic phases were combined and washed with saturated brine, the obtained ethyl acetate solution was dried over anhydrous sodium sulfate, filtered Remove the desiccant, and the residue obtained after vacuum distillation is separated by silica gel column chromatography (V petroleum et...
Embodiment 3
[0022] Example 3 2-(N-acetyl)-2[1-(2,4-dihydroxy)phenyl]propanoic acid
[0023] To a solution of methyl 2-acetamidoacrylate (199 mg, 1.39 mmol) in 10 mL of dry THF was added 0.8 mL of BF at room temperature 3 ·Et 2 O (3 mmol), after stirring for 0.5 hours, add 5 milliliters of resorcinol (130 mg, 1.19 mmol) in anhydrous tetrahydrofuran solution, continue the reaction at room temperature for 48 hours, distill off the solvent under reduced pressure, and use an appropriate amount of After ethyl acetate was dissolved, saturated sodium bicarbonate solution was added, and the layers were extracted. The aqueous phase was extracted three times with ethyl acetate. The organic phases were combined and washed with saturated brine. The obtained ethyl acetate solution was dried with anhydrous sodium sulfate, filtered and removed. agent, and the residue obtained after vacuum distillation was separated by silica gel column chromatography (V petroleum ether / V ethyl acetate 1 / 1) to obtain 2-(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com