Acipenser sinensis antibacterial peptide and preparation method and application thereof
An antibacterial peptide, Chinese sturgeon technology, applied in the field of genetic engineering, can solve the problem of not finding protein peptides and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
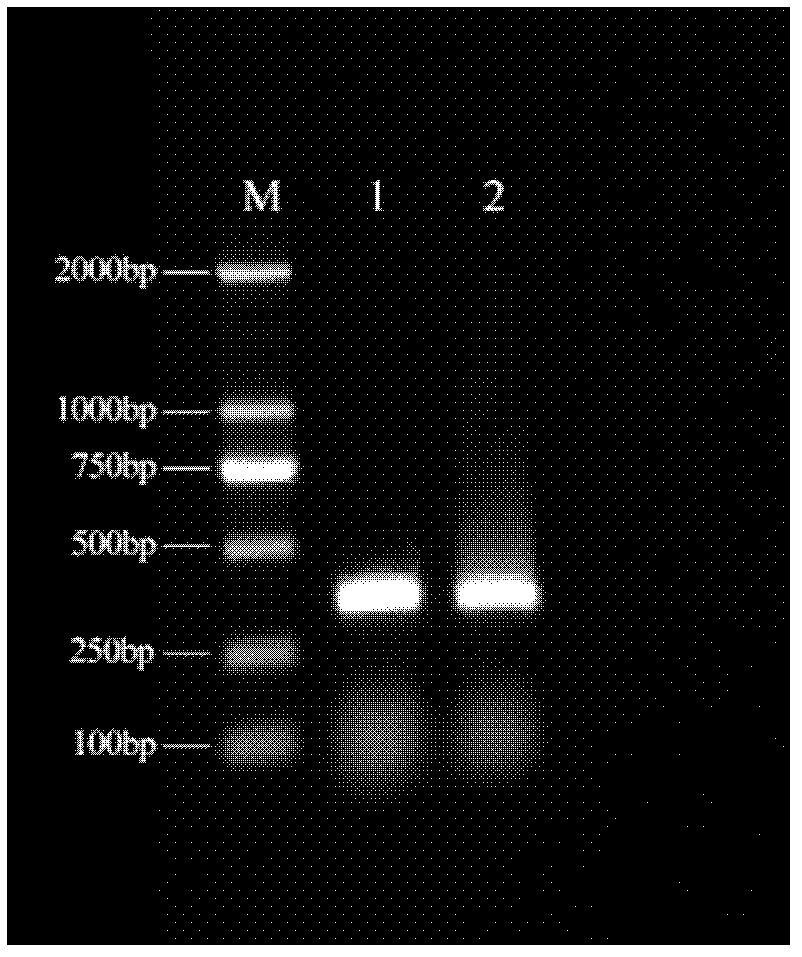
[0048] Example 1: Cloning of Chinese sturgeon antimicrobial peptide gene
[0049] (1) Primer design
[0050] According to the fish hepcidin sequence information registered in NCBI / GenBank, according to the conservation of the sequence, a pair of degenerate primers (F1, R1) were designed in the upstream and downstream regions of the cDNA sequence ORF with Primer 5.0 software; F1: 5'-ACAYCAGAACWAACAYTC- 3'
[0051] R1: 5'-CYACTTTTAYAAGGCWTA-3'.
[0052] (2) Extraction of blood total RNA by Trizol method
[0053] Draw blood from the caudal artery of Chinese sturgeon with a sterile disposable syringe. After standing at room temperature for a period of time, centrifuge at 1000 rpm for 30 minutes at room temperature, discard the supernatant, and precipitate to become blood cells.
[0054] The glassware used in the experiment was treated with 0.1% DEPC water and baked at 150°C for 4h. Plastic containers were soaked in 0.1% DEPC water overnight, and then sterilized under high temp...
Embodiment 2
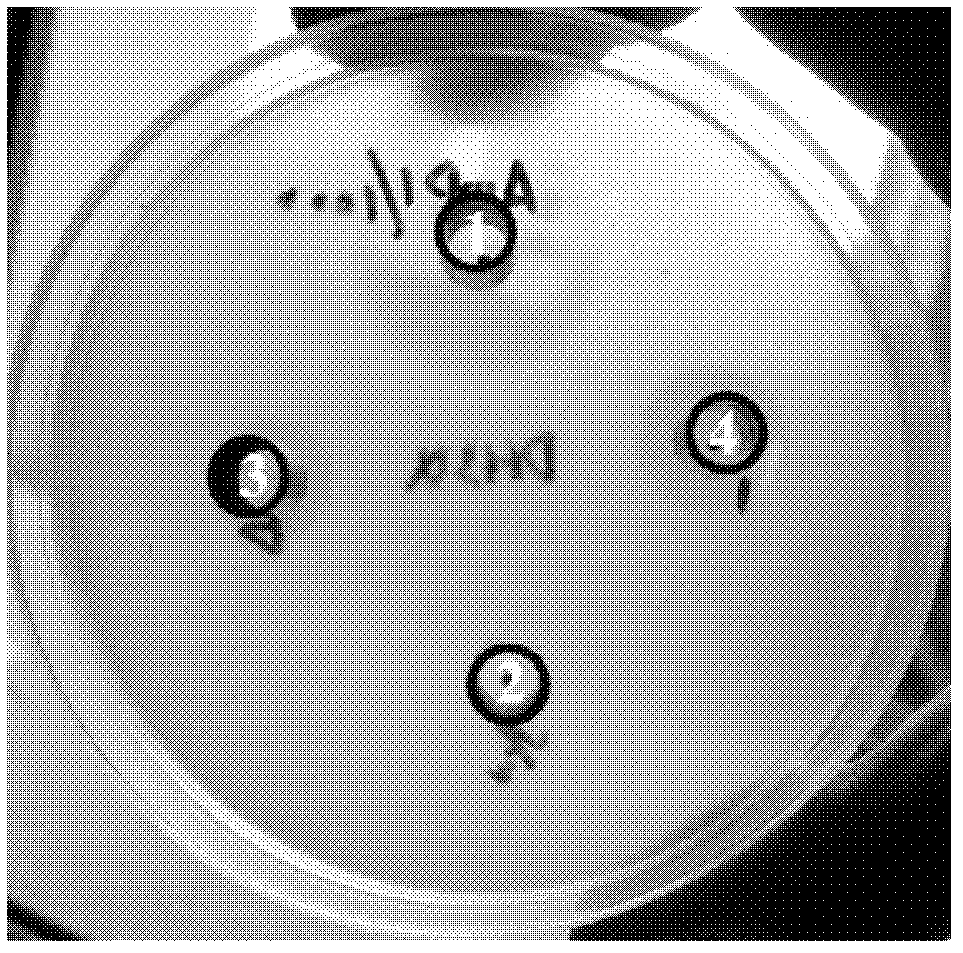
[0076] Example 2: Expression and antibacterial activity analysis of recombinant eukaryotic expression vector in Pichia pastoris
[0077] (1) Linearization of recombinant plasmids
[0078] Recombinant expression plasmid pPICZαA-pen-epcidin and pPICZαA empty vector were cut with restriction endonuclease BstX I respectively, the system is as follows:
[0079]
[0080] After reacting at 45°C for 5h, the digested product was purified with a DNA cleaning kit, eluted with 20 μL ddH2O, and stored at -20°C.
[0081] (2) Electrotransformation of yeast competent cells with linearized recombinant plasmids
[0082] Mix 80 μL of Pichia pastoris competent cells (Pichia pastoris) with BstX I linearized pPICZαA-antimicrobial peptide plasmid (20 μL), transfer to a pre-cooled 0.2 cm electroporation cup (Bio-Red), and place 5min on ice, 2kV, 25μF, 400Ω, after electric shock for 8 milliseconds, immediately add 1mL of pre-cooled 1mol / L sorbitol, take 200μL and spread them on YPDS containing Ze...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com