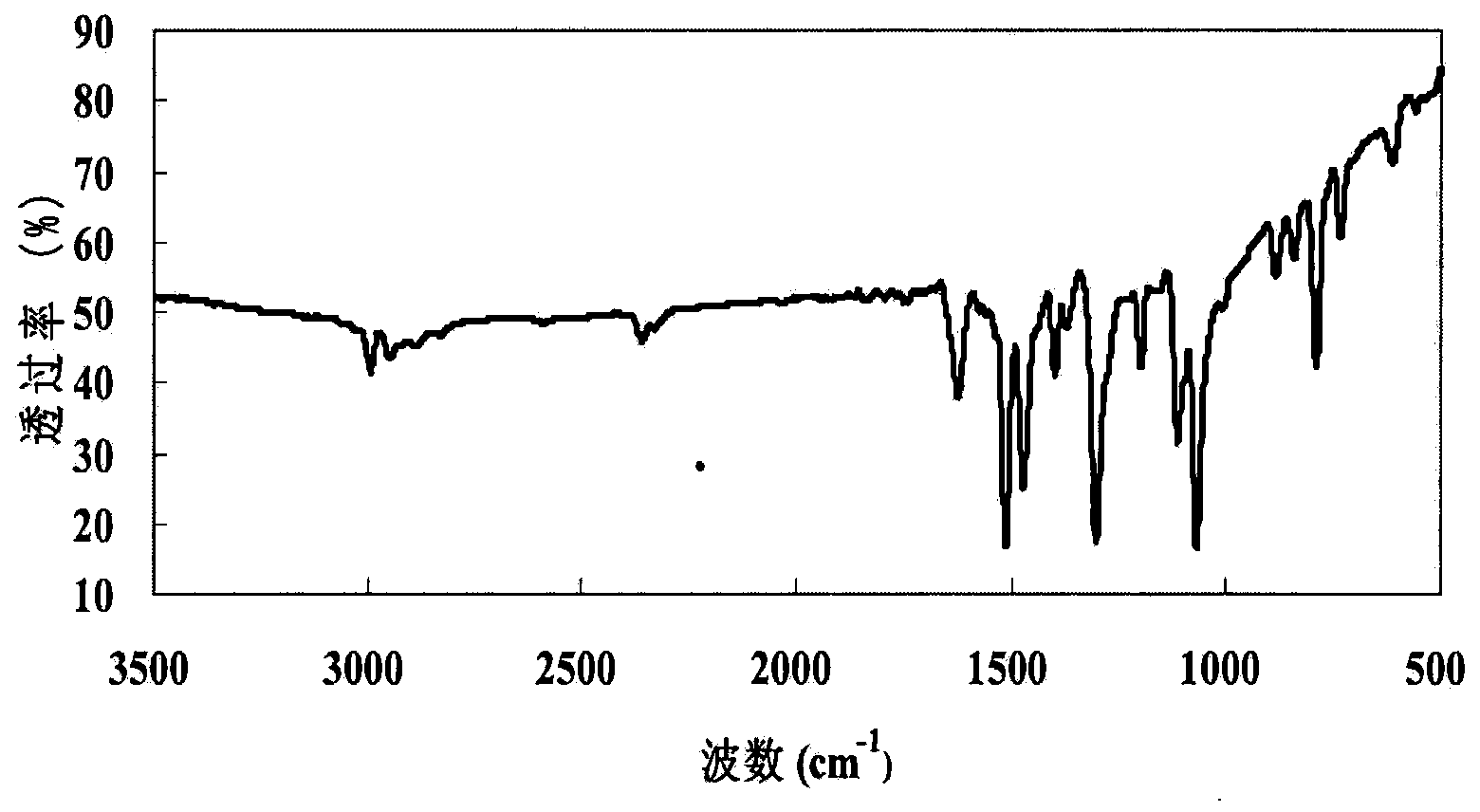
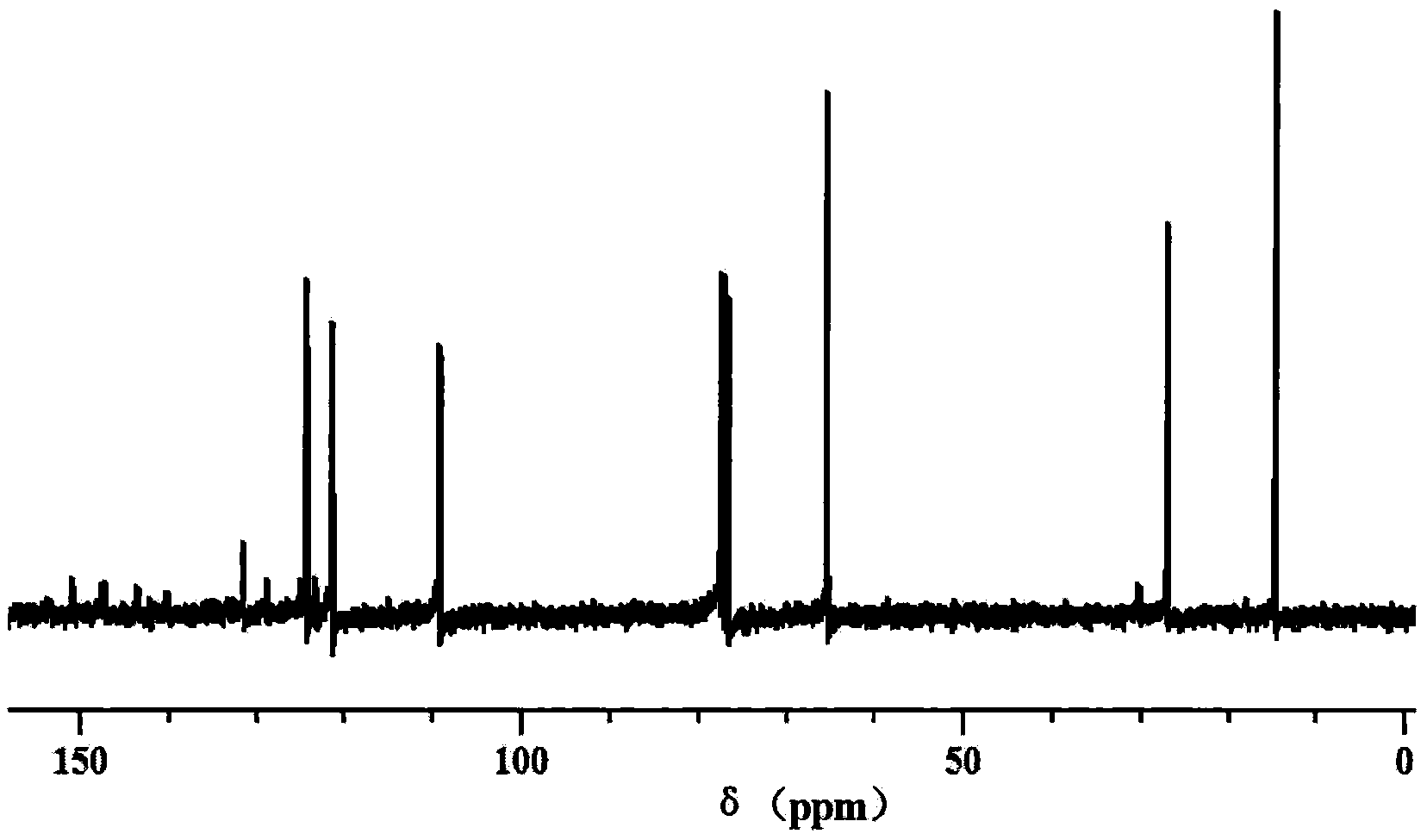
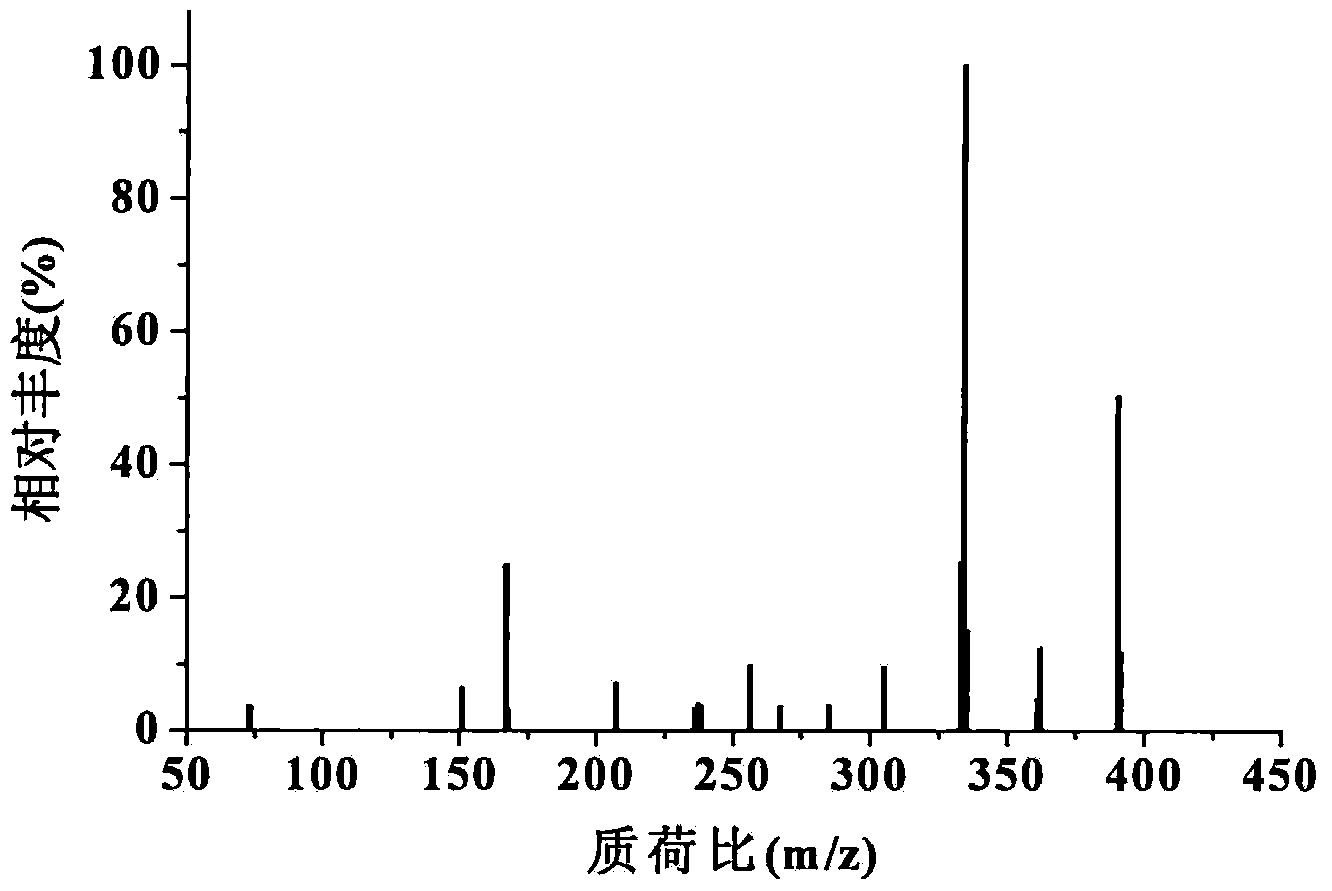
Cyclohexadiene polyfluoro liquid crystal compound and preparation method thereof
A liquid crystal compound, cyclohexadiene technology, applied in the field of materials, can solve the problems of increasing the dielectric anisotropy value, low solubility, large birefringence, etc., to improve the dielectric anisotropy, wide temperature range, improve The effect of speed of response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Taking the preparation of 1,4-bis-(2,3-difluoro-4-ethoxyphenyl)-1,4-cyclohexadiene as an example, the raw materials used and its preparation method are as follows:
[0031] 1. Preparation of 4-hydroxyl-4-(2,3-difluoro-4-ethoxyphenyl)cyclohexanone
[0032] Under nitrogen protection, add 3.46g (0.144mol) of magnesium powder, 50mg of iodine, and 50mL of ether into a 500mL three-necked flask, heat the oil bath to 30°C, and drop 28.44g (0.12mol) of diethyl ether dissolved in 50mL of ) 4-bromo-2,3-difluorophenetole, first add 5mL dropwise, after the reaction is triggered, then dropwise add the remaining solution, after the dropwise addition, heat up to 40°C, reflux for 1 hour, then dropwise add and dissolve in 150mL 15.60g (0.1mol) of diethyl ether 1,4-cyclohexanedione ethylene glycol monoketal, reflux reaction for 6 hours after the dropwise addition, cooled to room temperature, poured into 200mL of 15% hydrochloric acid aqueous solution, heated to 60°C, hydrolyze at constan...
Embodiment 2
[0045] Taking the preparation of 1,4-bis-(2,3-difluoro-4-ethoxyphenyl)-1,4-cyclohexadiene as an example, the raw materials used and its preparation method are as follows:
[0046] In step 1 of the preparation of 4-hydroxyl-4-(2,3-difluoro-4-ethoxyphenyl)cyclohexanone in this embodiment, the hydrolysis conditions are: hydrolysis at 20°C for 4 hours, other steps of this step are the same as Same as in Example 1, 8.85 g of 4-hydroxy-4-(2,3-difluoro-4-ethoxyphenyl)cyclohexanone was prepared, and the yield was 32.76%. Other steps were the same as in Example 1 to prepare 1,4-bis-(2,3-difluoro-4-ethoxyphenyl)-1,4-cyclohexadiene.
Embodiment 3
[0048] Taking the preparation of 1,4-bis-(2,3-difluoro-4-ethoxyphenyl)-1,4-cyclohexadiene as an example, the raw materials used and its preparation method are as follows:
[0049] In step 1 of the preparation of 4-hydroxyl-4-(2,3-difluoro-4-ethoxyphenyl)cyclohexanone in this embodiment, the hydrolysis conditions are: hydrolysis at 40°C for 4 hours, other steps of this step are the same as In the same manner as in Example 1, 18.28 g of 4-hydroxy-4-(2,3-difluoro-4-ethoxyphenyl)cyclohexanone was prepared, with a yield of 67.68%. Other steps were the same as in Example 1 to prepare 1,4-bis-(2,3-difluoro-4-ethoxyphenyl)-1,4-cyclohexadiene.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com