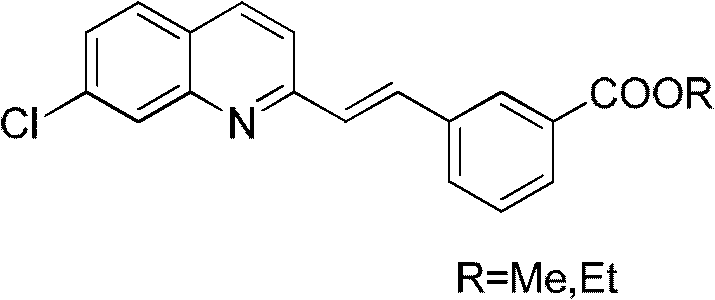
Compounding method of midbody required in compounding of montelukast sodium
A technology for the synthesis of montelukast sodium and its synthesis method, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of high production equipment requirements and high prices, and achieve the effect of simple raw materials, mild conditions, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
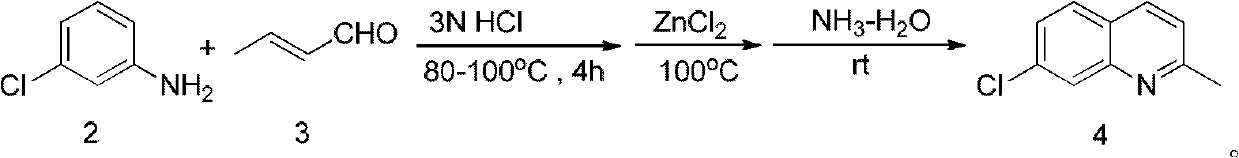
[0038] 250mL three-necked bottle, equipped with a reflux condenser, a mechanical stirring paddle, and nitrogen protection. At room temperature, add 40 mL of 3N hydrochloric acid and 40 mL of ethanol to the bottle, and stir. Add 10.4 mL of m-chloroaniline and stir to form a white slurry. Heat the oil bath to 85-100°C, add 10.32 mL of crotonaldehyde dropwise, the system turns into a yellow turbid liquid, and reflux for 2 hours. Cool to room temperature, extract twice with 40 mL of petroleum ether, and discard the organic phase. The obtained aqueous phase was put into a 250mL three-neck flask equipped with a stirring paddle and a reflux condenser, stirred at a high speed at room temperature, and 13.6g of zinc chloride was added. The oil bath was heated to 120° C. and refluxed for half an hour. Keep stirring at a high speed, cool to room temperature, a precipitate appears, and the mother liquor is nearly colorless and transparent. The mother liquor was discarded, and the obtai...
Embodiment 2
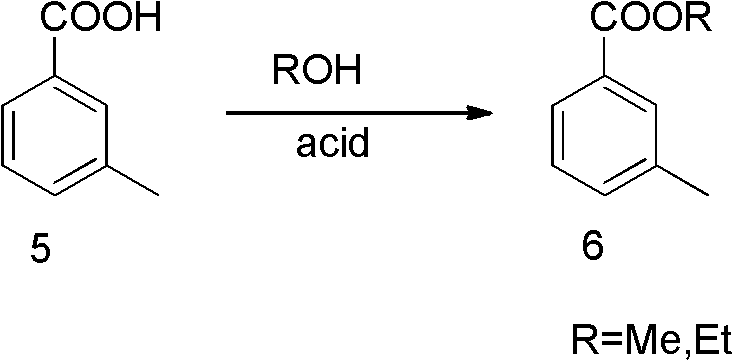
[0041]250mL three-neck bottle, install the reflux water separation device. At room temperature, add 30 g of m-toluenesulfonic acid, 5 g of p-toluenesulfonic acid (or 2.16 g of concentrated sulfuric acid), 40 mL of ethanol, and 90 mL of benzene into the bottle, and stir to form a colorless and clear liquid. Heat the oil bath to 120°C, reflux and separate water for about 16 hours. Cool to room temperature, distill off benzene and excess ethanol to obtain brown viscous turbid liquid, add 100mL of ethyl acetate to dissolve, wash with 30mL of water, 30mL of saturated sodium bicarbonate solution, 30mL of saturated sodium chloride solution, and separate The ethyl acetate phase was dried over anhydrous sodium sulfate, and the solvent was spun off to obtain a yellow clear viscous liquid. Distilled under reduced pressure to obtain compound 6 as a colorless clear viscous liquid with a special smell, with a yield of 86%.
[0042] 1 H NMR (CDCl 3 ) δ (ppm): 1.40 ~ 1.44 (t, 3H); 2.43 (s...
Embodiment 3
[0044] 250mL three-necked bottle, install a reflux condenser. At room temperature, 8.2 g of compound (6), 160 mL of carbon tetrachloride, and 9 g of NBS were added to the bottle, and stirred evenly to form a light yellow suspension. Heat the oil bath to 80°C for reflux. After reflux, use a 200W light bulb to irradiate at a distance of 2 cm from the reaction bottle. At the same time, lower the temperature of the oil bath to keep the reaction system gently refluxed for 16 hours. Sampling TLC showed disappearance of starting material with an apparent spot of new material. Turn off light and cool to room temperature. Filter out the precipitate with diatomaceous earth, and wash the obtained clear water with 50 mL*2 of water and 50 mL of saturated sodium chloride solution in sequence. The organic phase after liquid separation was dried with anhydrous sodium sulfate, distilled under reduced pressure, and the solvent was recovered to obtain a yellow-brown oily liquid. Distilled und...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com