Rare earth complex nanobelt and preparation method thereof
A technology of rare earth complexes and nanobelts, applied in the field of nanomaterials, can solve the problems that limit the development and application of rare earth complexes, and achieve the effects of strong rare earth ion characteristic luminescence, uniform shape and size, and good dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The invention discloses a preparation method of a rare earth complex nanobelt, comprising the following steps: mixing RE nitrate and phthalic acid (1,2-BDC) in a polar solvent to obtain a mixed solution, RE being Gd or Y; add ammonia (NH 3 ·H 2 O), adjusting the pH value to 5.5-6.5, and reacting at 70-90° C. to obtain nanobelts of rare earth complexes.
[0033] In the above preparation process, the present invention reacts the rare earth nitrate with phthalic acid to deprotonate the carboxyl group of phthalic acid, realizes the coordination combination of rare earth ions and phthalic acid, and produces a large number of crystal nuclei. The crystal nucleus grows anisotropically in a polar solvent environment, and the chemical formula is RE 4 (1,2-BDC) 6 (H 2 O) 2 ·nH 2 O nanobelts of rare earth complexes.
[0034] Because rare earth elements have rich coordination modes and geometric configurations, they can produce rich topological structures, and their unique 4f...
Embodiment 1
[0053] Weigh phthalic acid (1,2-H 2 2.5 grams of BDC) was placed in a flask, 500 mL of deionized water was added, and 1 mol·L -1 Gd(NO 3 ) 3 solution 10mL, add NH dropwise 3 ·H 2 O adjusts the pH of the solution to 6, reacts for 1 hour, washes repeatedly with ethanol and deionized water, collects after centrifugation, and dries in the air to obtain the final product, that is, nanobelts of rare earth complexes, which are composed of Gd 4 (1,2-BDC) 6 (H 2 O) 2 4H 2 O.
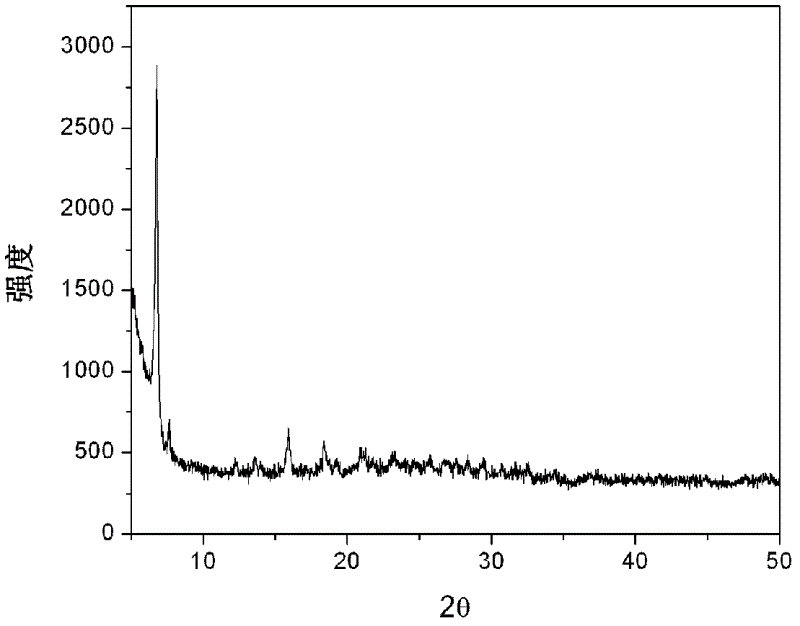
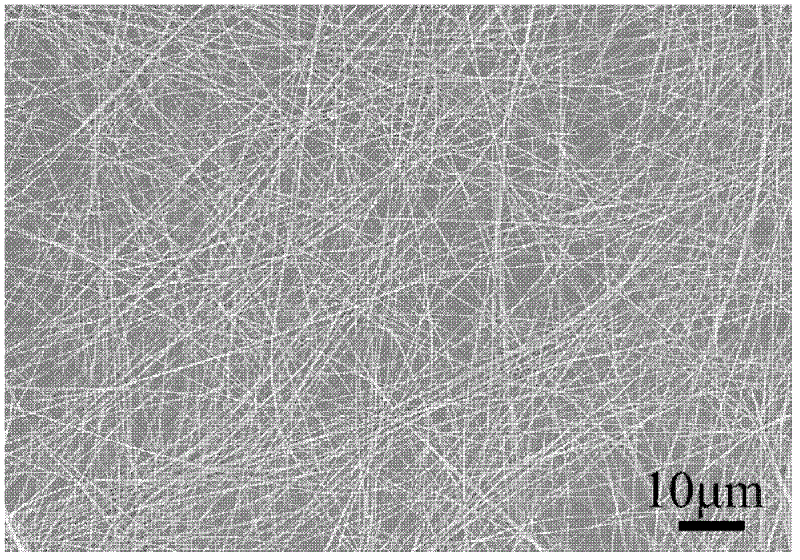
[0054] The rare earth complex nanoribbons prepared in this example were subjected to XRD pattern analysis and scanning electron microscope analysis respectively, figure 1 The XRD pattern of the rare earth complex nanobelt prepared for the present embodiment, figure 2 SEM photos of the rare earth complex nanoribbons prepared for this example. It can be seen from the figure that the rare earth complex nanoribbon obtained in this example has a length of more than several hundred micrometers, uniform shap...
Embodiment 2
[0056] Weigh phthalic acid (1,2-H 2 2.5 grams of BDC) was placed in a flask, 500 mL of deionized water was added, and 1 mol·L -1 Gd(NO 3 ) 3 Solution 10mL and Eu(NO 3 ) 3 Solution 0.05mmoL, drop NH 3 ·H 2 O adjusts the pH of the solution to 6, reacts for 1 hour, washes repeatedly with ethanol and deionized water, collects after centrifugation, and dries in the air to obtain the final product, that is, nanobelts of rare earth complexes, which are composed of Gd 4 (1,2-BDC) 6 (H 2 O) 2 4H 2 O:0.5%Eu 3+ .
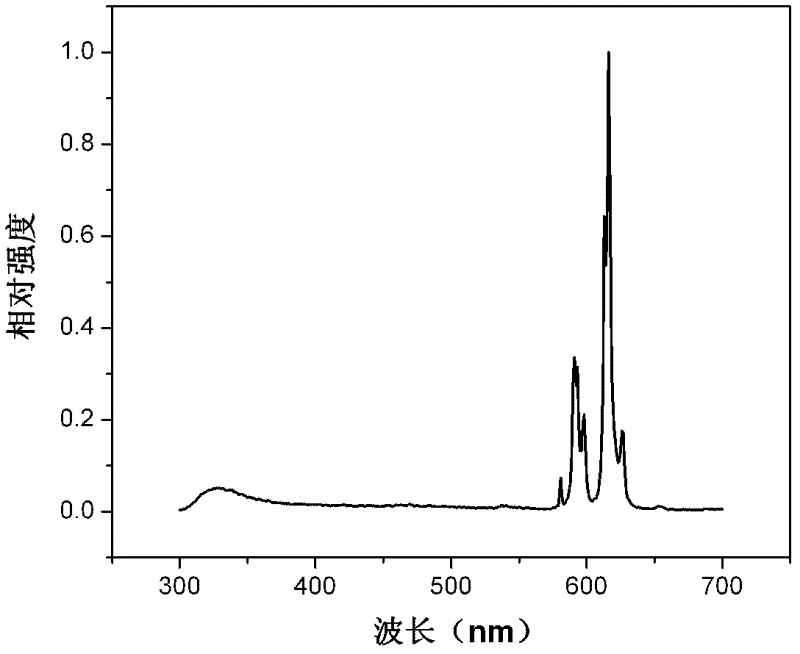
[0057] The rare earth complex nanoribbons prepared in this example were tested for photoluminescence performance, such as image 3 Shown is the photoluminescence emission spectrum of the rare earth complex nanobelt prepared in this example under the excitation condition of 280nm ultraviolet light. It can be seen from the figure that the rare earth complex nanoribbon prepared in the embodiment of the present invention shows strong characteristic luminescence of rar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Width | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com