Maleimide-polyglutamic acid-aspartic acid polymer and composite thereof, preparation methods for maleimide-polyglutamic acid-aspartic acid polymer and composite thereof, and application of maleimide-polyglutamic acid-aspartic acid polymer and composite thereof
A technology of maleimide and maleimide group, applied in the field of biomedicine, can solve the problems of lack of active targeting function and inability to directly couple ligands, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1 Preparation of acid type γ-polyglutamic acid-aspartic acid
[0081] Gamma-polyglutamic acid was prepared by fermentation of Bacillus licheniformis ATCC 9945a, and the reference [Haifeng Ye et al. Biomaterial 27 5958-5965] was slightly modified. Inoculate the fermentation medium (maltose 50 g l -1 , yeast extract 10 g·l -1 , sodium glutamate 30 g·l -1 , NaCl 10g·l -1 , KH 2 PO 4 5g·l -1 ,MgSO 4 ·7H 2 O 0.5g·l -1 ), 37 0 C, 220 rpm -1 Shake flasks were incubated for 72 hours. After the fermentation is finished, dilute the fermentation broth 4 times with distilled water, adjust the pH of the fermentation broth to 2.0-3.0 with hydrochloric acid, 12000 r min -1, Centrifuge for 30 minutes to remove the bacterial precipitate, then adjust the pH of the supernatant to 7.0-8.0, then add 3 times the volume of ice-cold absolute ethanol, stir to obtain the γ-polyglutamic acid precipitate, and re-dissolve the precipitate in distilled water, Remove insolub...
Embodiment 2
[0085] Embodiment 2 The preparation method of maleimide-polyglutamic acid-aspartic acid polymer
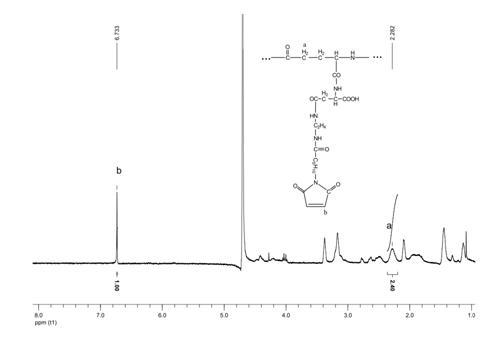
[0086] Reaction scheme 1:
[0087] Take the acid-type γ-polyglutamic acid-aspartic acid (PGA-Asp, 37 mg) prepared in Example 1 above, dissolve it in dimethyl sulfoxide solution (DMSO, 10 ml), add 1-hydroxybenzene Triazole (HOBt, 80mg) and 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC, 120mg), drop 150 μL of triethylamine and N-(2 -Aminoethyl)-6-maleimidocaproamide solution (400 mg, dissolved in 1 ml DMFO), stirred at 37°C under nitrogen protection for 72 hours. Add 15 times the volume of diethyl ether to the reaction system solution to terminate the reaction, shake vigorously, the mixed solution is divided into upper and lower layers, and the lower layer liquid is light yellow. The lower liquid was collected, added an equal volume of phosphate buffer (pH 7.0, 0.2M), dialyzed for 48 hours, and freeze-dried to obtain a white floc of maleimide-polyglutamic acid-...
Embodiment 3
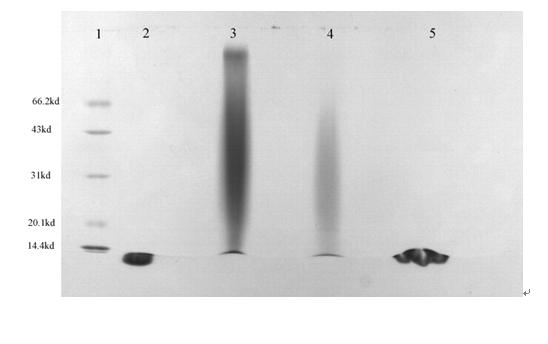
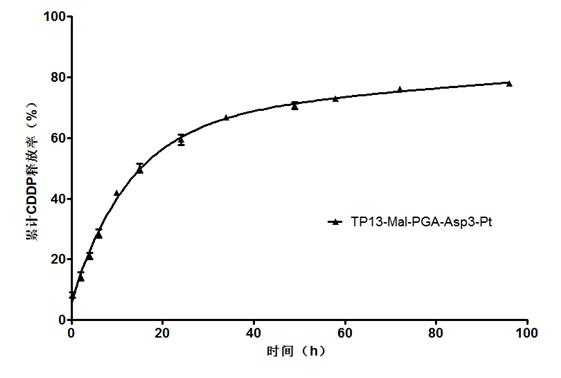
[0118] Example 3 Preparation of IFN maleimide-polyglutamic acid-aspartic acid complex
[0119] Dissolve the maleimide-polyglutamic acid-aspartic acid polymer (Mal-PGA-Asp1, 48 mg) prepared in reaction scheme 1 of Example 2 in deionized water (10 ml), add recombinant human interference IFN α-2b (IFN α-2b, 10mg), react at 4°C for 12 hours. This preparation condition is conducive to retaining the activity of recombinant human interferon α-2b. It is separated by dialysis, ultrafiltration and size exclusion chromatography. Purification, the obtained IFN-maleimide-polyglutamic acid-aspartic acid complex, the general structural formula is:
[0120]
[0121] Among them, n=51 (average value); m=42 (average value); z=21 (average value); Mal is N-(2-aminoethyl)-6-maleimide caproamide; γ- PGA is γ-polyglutamic acid; Asp is aspartic acid; IFN is recombinant human interferon α-2b (IFN α-2b).
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com