Construction method for HPLC (high performance liquid chromatography) finger-print chromatogram of ginseng and astragalus strengthening injection and application of finger-print
A technology of Shenqi strengthening and fingerprinting, applied in the field of drug analysis, can solve the problems of overlapping chromatographic peak information, affecting method reproducibility, and less chromatographic peak attribution, etc., to achieve rich chemical information, good specificity, and reproducibility. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
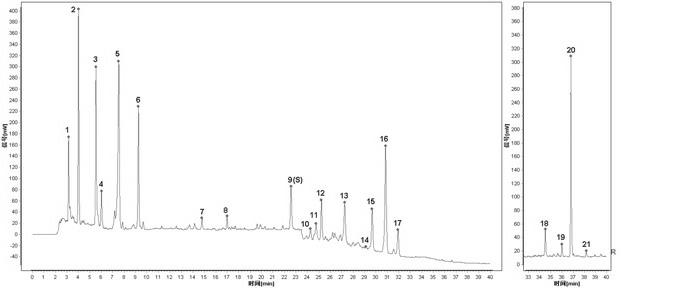
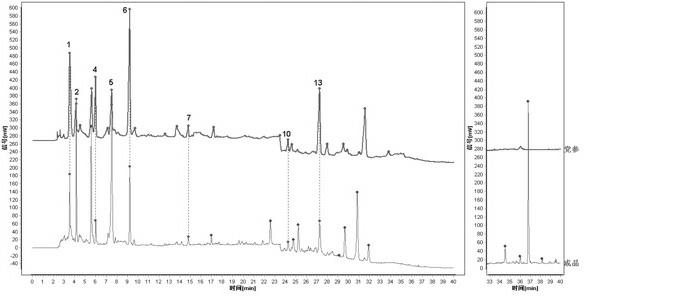
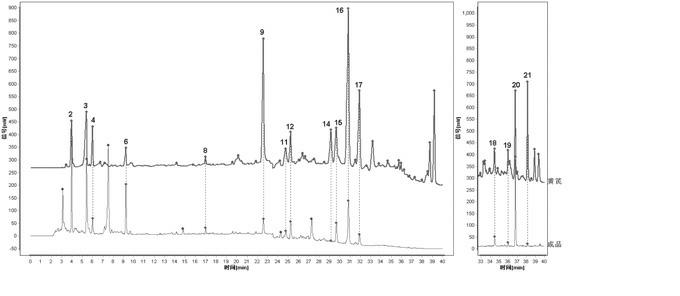
[0032] Embodiment 1: The construction method of HPLC fingerprint of Shenqi Fuzheng Injection and the standard fingerprint of Shenqi Fuzheng Injection
[0033] 1 Instruments and reagents
[0034] 1.1 Instruments: Dionex Ultimate 3000 DGLC high performance liquid chromatography (DGP-3600SD double ternary pump, SRD-3600 degasser, WPS-3000SL autosampler, TCC3000-RS column thermostat, DAD detector) , Chromeleon6.8 data processing software), ELSD detector (French SEDERE company, SEDEX 75 type); pretreatment chromatographic column: Dionex Acclaim? Polar Advantage C18 (3μm, 50 mm×3.0mm); analytical chromatographic column: Agilent Agilent ZORBAX Eclipse Plus C18 (3.5 μm, 150 mm × 3.0 mm).
[0035] 1.2 Test drug: 10 batches of finished products and control medicinal material extracts were provided by Limin Pharmaceutical Factory of Livzon Pharmaceutical Group. The acetonitrile reagent used in the liquid chromatography in the experiment is chromatographically pure, the rest of the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com