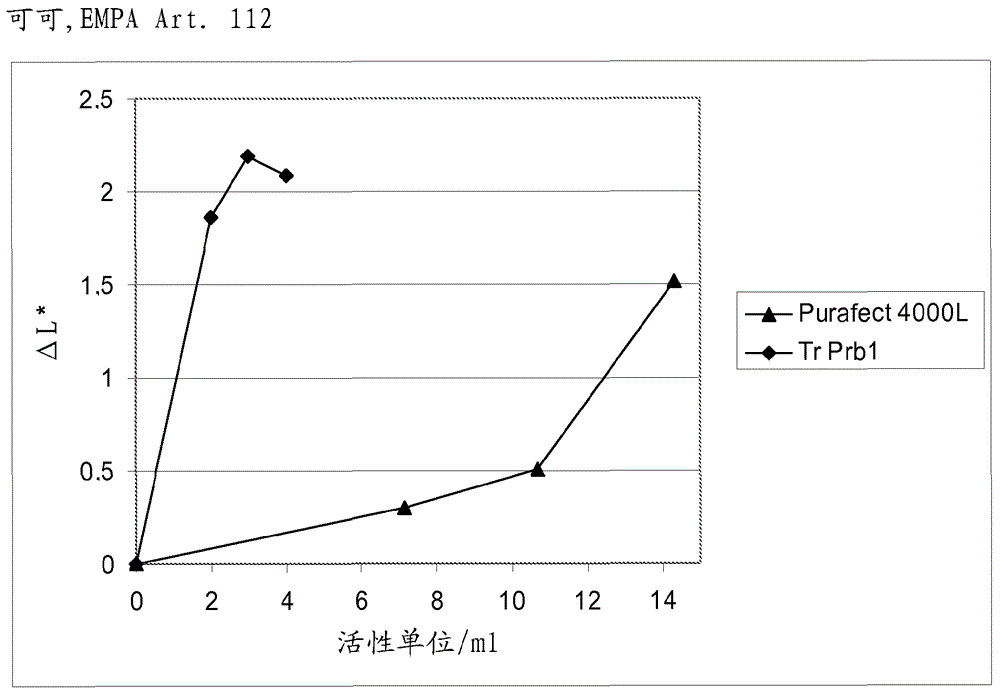
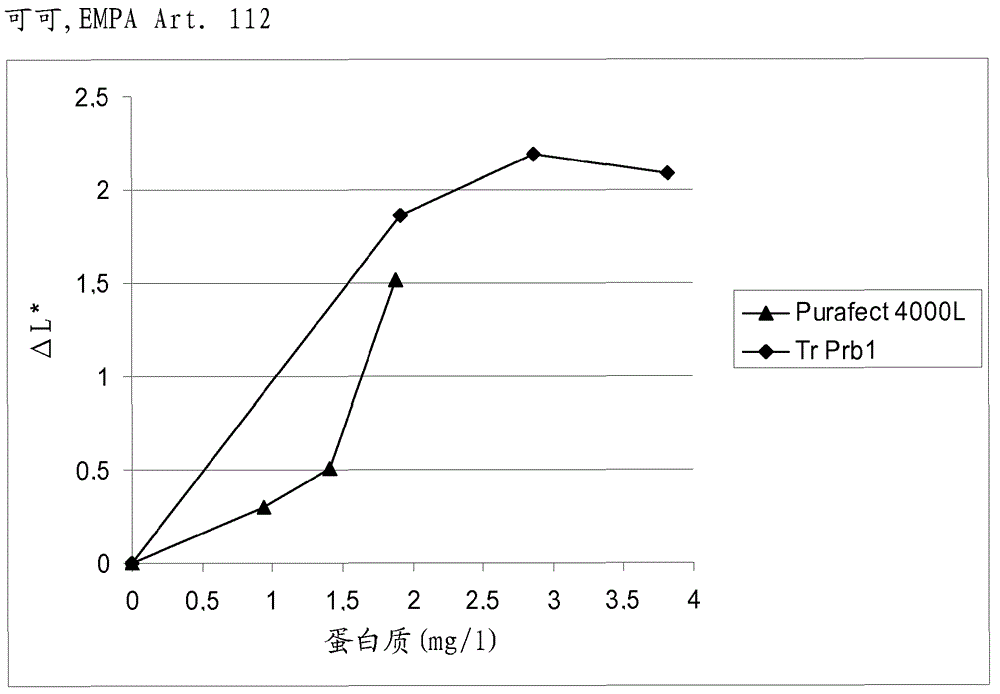
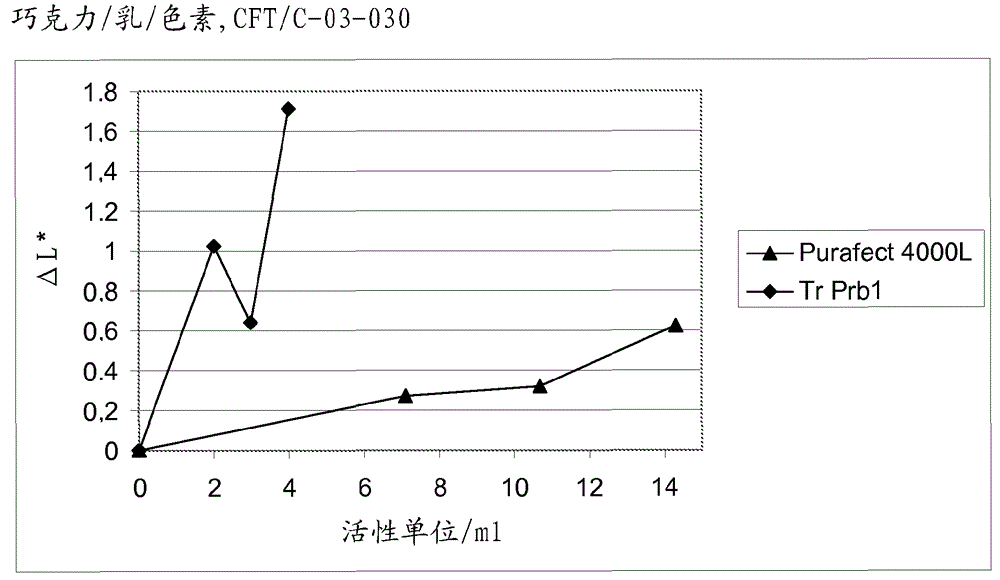
A fungal protease and use thereof
A serine protease, fungus technology, applied in the direction of hydrolase, biochemical treatment of enzymes/microorganisms, introduction of foreign genetic material using vectors, etc., can solve problems such as unresearched
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0209] Example 1. Trichoderma reesei QM6a prb1 ( Tr prb1 ) And Fusarium graminearum ALKO1726 Fg prtS8A Cloning of protease gene
[0210] (A) Molecular biology methods for DNA isolation and use
[0211] Standard molecular biology methods are used for DNA isolation and enzymatic treatment (eg isolation of plasmid DNA, digestion of DNA to produce DNA fragments), transformation in E. coli, sequencing, etc. The basic method used is as described by the manufacturer of the enzyme, reagent or reagent expression kit, or as described in standard molecular biology manuals such as Sambrook and Russell (2001). The isolation of genomic DNA from Trichoderma reesei QM6a and Fusarium graminearum ALKO1726 was completed as described by Raeder and Broda (1985). A PhaseLock tube (Eppendorf, Germany) was used to perform the phenol extraction stage.
[0212] (B) Oligonucleotide primers for gene cloning
[0213] Using Trichoderma reesei QM6a and Fusarium graminearum ALKO1726 genomic DNA preparations as ...
Embodiment 2
[0223] Example 2. Production of Tr Prb1 and Fg_ALKO1726 recombinant proteases in Trichoderma reesei
[0224] (A) Preparation of expression cassette and its transformation into Trichoderma reesei
[0225] By separately amdS ( The acetamidase) marker gene was ligated into the plasmids pALK2650 and pALK2707 (Example 1c) to construct the expression plasmid pALK2701 (for the production of recombinant Tr Prb1 and Fg_ALKO1726 proteins in Trichoderma reesei). Tr prb1 ) And pALK2708 ( Fg prtS8A ). amdS Mark at cbh1 The terminator is then connected to the expression construct. Similar constructions have been described in, for example, Paloheimo et al. (2003). In the expression cassettes pALK2701 and pALK2708 ( image 3 with 4 ), through PCR will have its own signal sequence Tr prb1 with Fg prtS8A Genes and Trichoderma reesei cbh1 (cel7A) The promoter is exactly fused. Use generated after the stop codon in PCR Bam HI site, connect the 3-' end of the gene with cbh1 Terminator fusion...
Embodiment 3
[0231] Example 3. Purification and characterization of recombinant Tr Prb1 and Fg_ALKO1726 protease
[0232] Cells and solids were removed from the depleted medium obtained from the fermentation (Example 2) by centrifugation at 4°C for 30 minutes, 50,000 g (Sorvall RC6 plus). 15 ml of supernatant was used for the purification of protease. All purification steps are performed in the cold room. After centrifugation, the sample was filtered through a 0.44 μm filter (MILLEX HV Millipore) before being applied to a HiPrep 26 / 10 Desalting column (from GE Healthcare) equilibrated in 20 mM Tris pH 8.8. The gel-filtered samples were applied to a 20 mL Q Sepharose FF column (from GE Healthcare) equilibrated in 20 mM Tris pH 8.8. An Amicon Ultra centrifugal filter unit 10000 MWCO (Millipore) was used to concentrate the effluent fraction with proteolytic activity. The concentrated sample was applied to a Superdex 75 10 / 300 GL column (GE Healthcare) and eluted with 20 mM Hepes, 150 mM NaCl ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com