Preparation method for nano titanium dioxide lithium ion battery cathode material
A nano-titanium dioxide and lithium-ion battery technology, applied in the direction of titanium dioxide, battery electrodes, titanium oxide/hydroxide, etc., can solve problems such as long reaction cycle, difficult control of hydrolysis speed, complex reaction mechanism, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The invention provides a preparation method of nano-titanium dioxide lithium ion battery negative electrode material, comprising:
[0031] a) dissolving the titanium source compound in a mixed solution of acetic acid and ethanol; obtaining a first reaction solution;
[0032] b) mixing and dissolving oxalic acid in ethanol to obtain a second reaction solution;
[0033] c) mixing the first reaction solution with the second reaction solution, reacting oxalic acid with a titanium source compound to generate a precipitate;
[0034] d) roasting the precipitate obtained in the step c) to obtain the nano-titanium dioxide lithium ion battery negative electrode material.
[0035] Nano-titanium dioxide is a material that can replace carbon materials as the negative electrode of lithium-ion batteries. Since nano-titanium dioxide is prepared as a gel material, it needs to be dried when preparing the negative electrode material, and the drying time is long, which affects the use of ...
Embodiment 1
[0047] This example is used to illustrate the preparation of the silicon / carbon composite negative electrode material for lithium ion batteries provided by the present invention.
[0048] (1) Dissolve 5 mL of glacial acetic acid in 500 mL of ethanol solution, then add 6 mL of tetrabutyl titanate dropwise, and stir evenly to prepare a solution.
[0049] Add 1.89g of oxalic acid into a mixed solution of 30mL of water and 30mL of ethanol, and stir to prepare solution b.
[0050] (2) Put the solution b in a water bath at 50°C, and add the solution a dropwise, and stir for 3 hours. Aging at room temperature.
[0051] (3) Wash the obtained precipitate with ethanol and water respectively, and sinter at 400°C for 4h.
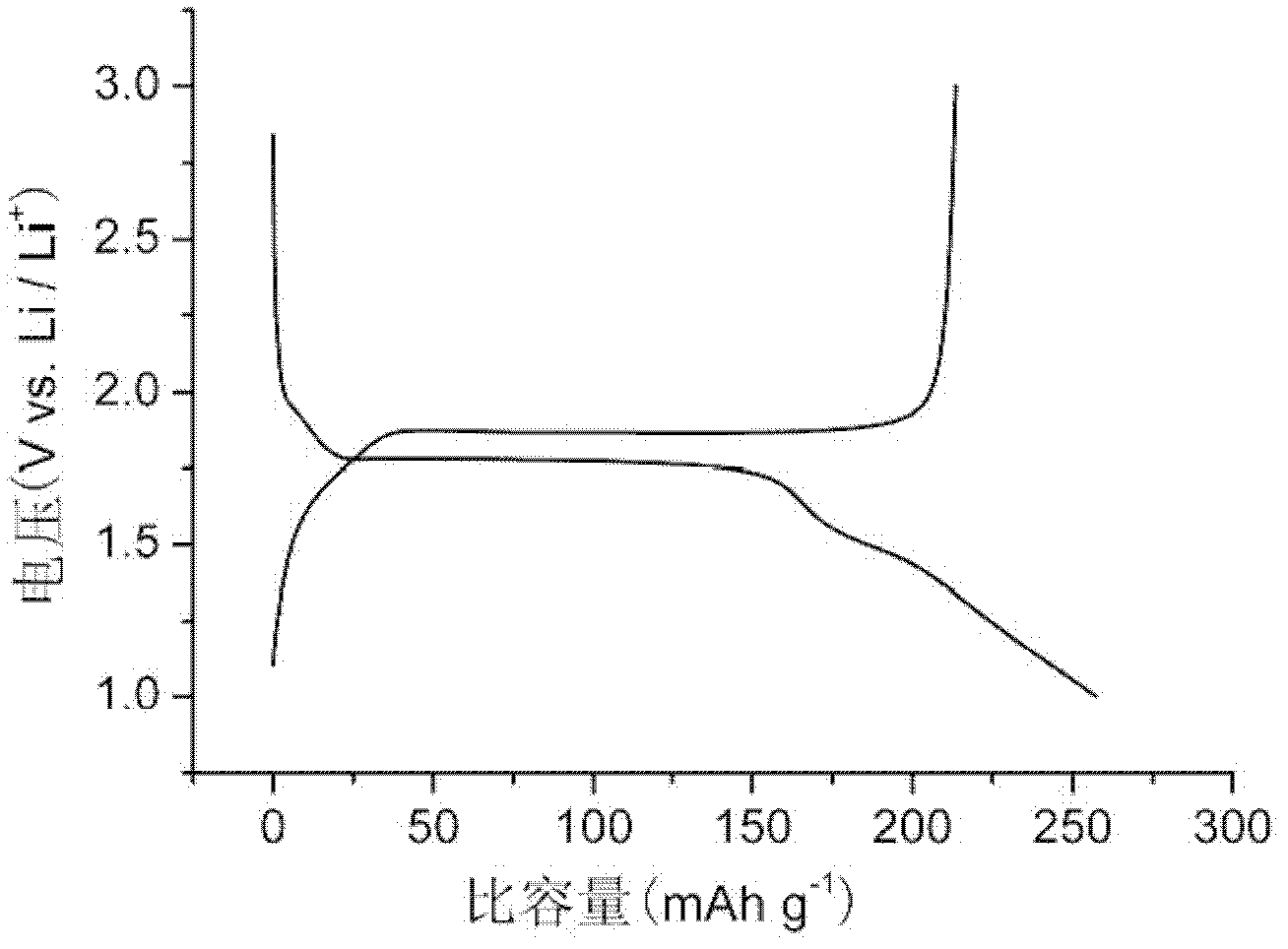
[0052] The obtained product has a lithium intercalation capacity of 257mAh / g for the first time, and a lithium delithiation capacity of 213mAh / g for the first time. See attached figure 1 .
Embodiment 2
[0054] (1) Dissolve 0.2mL of glacial acetic acid into 18mL of ethanol solution, then add 5mL of tetrabutyl titanate dropwise, and stir evenly to prepare a solution.
[0055] Add 2.4g of oxalic acid and 0.6g of sodium dodecylbenzenesulfonate to a mixed solution of 30mL of water and 30mL of ethanol, and stir to prepare solution b.
[0056] (2) Put the solution b in a water bath at 70°C, and add the solution a dropwise, and stir for 5 hours. Aging at room temperature.
[0057] (3) Wash the obtained precipitate with ethanol and water respectively, and sinter at 450°C for 4h.
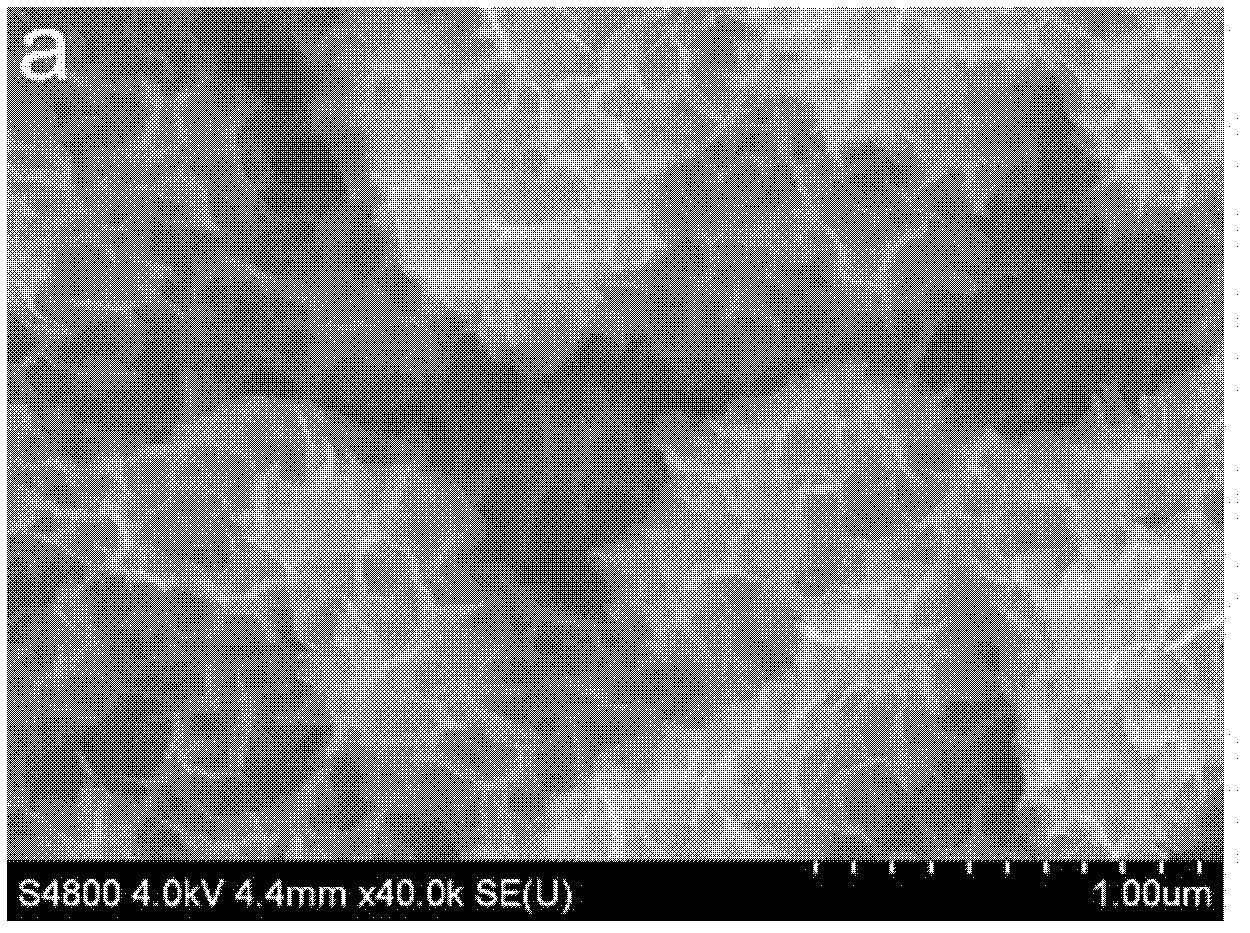
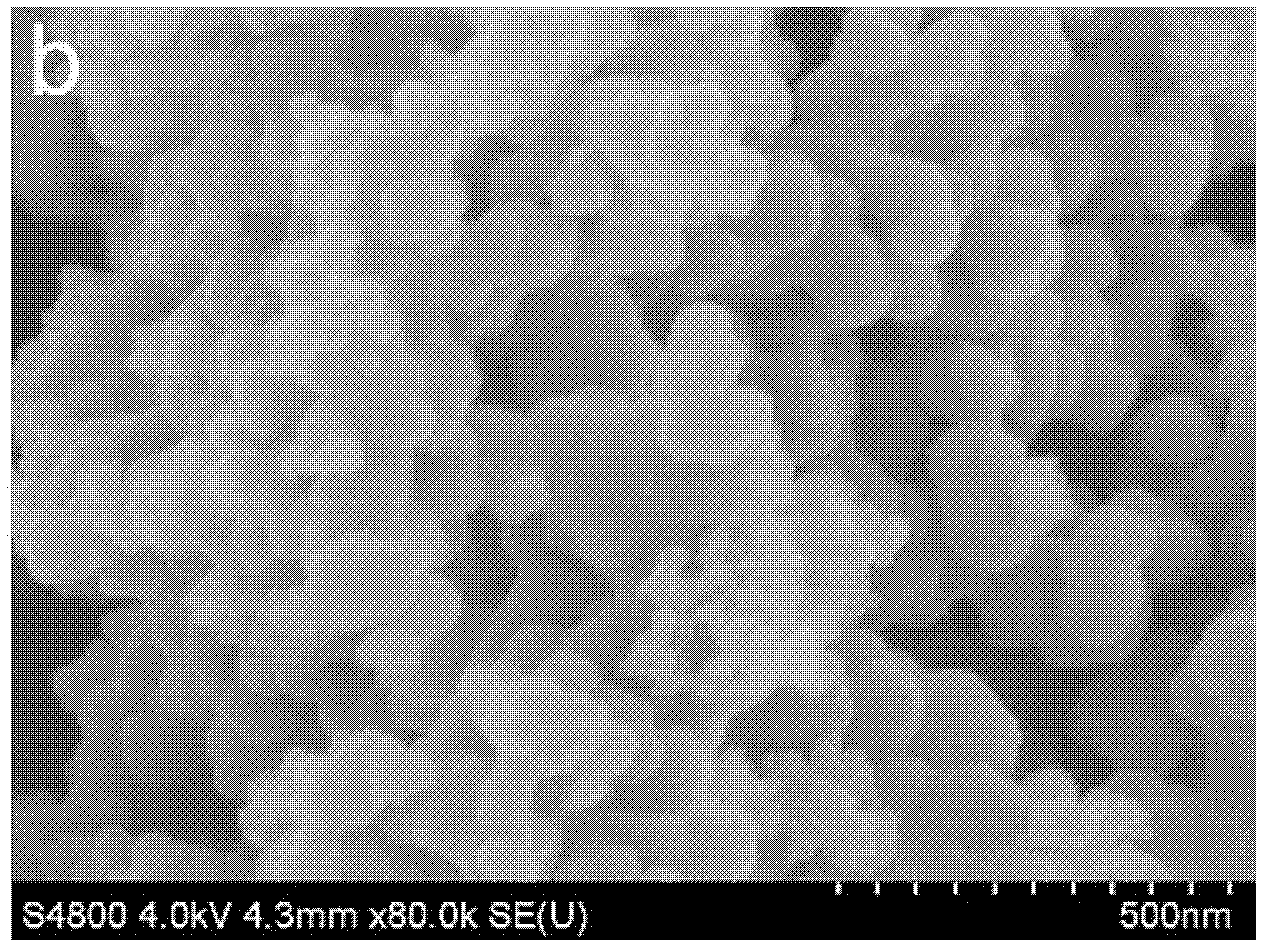
[0058] The morphology of the obtained product is shown in the appendix figure 2 . After 100 cycles at 1C, the capacity basically does not decay. See attached image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com