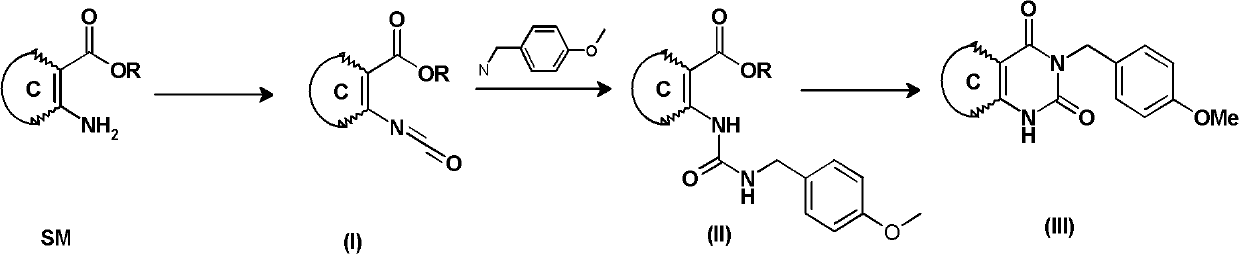
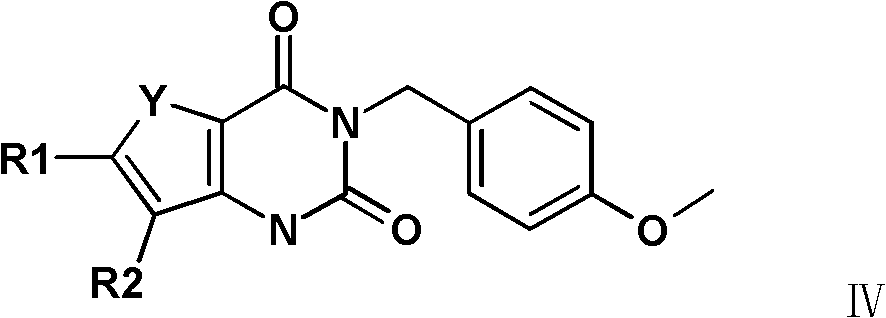
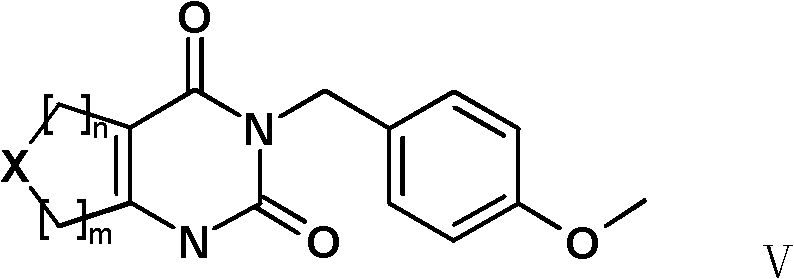
Synthetic method of 3-(4-methoxy-benzyl)-1H-pyrimidine-2,4-dione derivative
A technology of methoxybenzyl and synthetic method, which is applied in the field of organic synthesis, can solve the problems of low yield of target product, impossibility of reaction, low yield, etc., and achieve the effect of product purification ease and yield advantage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1 Synthesis of 3-(4-methoxybenzyl)-1H-thiophene[3,2-d]pyrimidine-2,4-dione (3) (method 1)
[0063]
[0064] 3-Amino-thiophene-2-carboxylic acid methyl ester (1) (4.7g, 30mmol) was added to dichloromethane (100ml) and saturated aqueous sodium bicarbonate (135ml) in a 500ml reaction flask at 0°C , forming a two-phase mixed system. Then slowly drop 20% phosgene toluene solution (16.9 ml, 34.5 mmol) into the mixed system with a syringe. After the dropwise addition was complete, the reaction mixture was stirred at 0° C. for an additional 15 minutes, then warmed to room temperature (20° C.) and stirred for an additional 1 hour. After the two phases were separated, the aqueous phase was extracted with 25 ml of dichloromethane, and the combined organic phase was dried over anhydrous magnesium sulfate, filtered, concentrated by rotary evaporation to about 30 ml, and the preparation of the isocyanate (2) dichloromethane solution was completed.
[0065] Dilute the dic...
Embodiment 2
[0069] Example 2 Synthesis of 3-(4-methoxybenzyl)-1H-thiophene[3,2-d]pyrimidine-2,4-dione (method 2)
[0070] Except using the toluene solution of 3.4g (11.5mmol) trimeric phosgene to replace 20% phosgene toluene solution (16.9ml, 34.5mmol) in embodiment 1, be used for other reaction process and treatment method of synthetic target compound and Example 1 is the same. 7.4 g of the target compound (3) was obtained with a yield of 86%. LC-MS and 1 The H-NMR analysis spectrum is the same as in Example 1.
Embodiment 3
[0071] Example 3 Synthesis of 6-bromo-3-(4-methoxybenzyl)-1H-thiophene[3,2-d]pyrimidine-2,4-dione (6)
[0072]
[0073] The reaction raw material 3-amino-5-bromothiophene-2-carboxylic acid methyl ester (4) was prepared according to the method cited in patent WO2010 / 101302A1. At 0°C, in a 500ml reaction flask, 3-amino-5-bromothiophene-2-carboxylic acid methyl ester (4.7g, 20mmol) was added to dichloromethane (85ml) and saturated aqueous sodium bicarbonate (100ml) , forming a two-phase mixed system. Then slowly drop 20% phosgene toluene solution (12.0 ml, 23 mmol) into the mixed system with a syringe. After the dropwise addition was complete, the reaction mixture was stirred at 0° C. for an additional 15 minutes, then warmed to room temperature (20° C.) and stirred for an additional 1 hour. After the two phases were separated, the aqueous phase was extracted with 20 ml of dichloromethane, and the combined organic phase was dried over anhydrous magnesium sulfate, filtered, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com