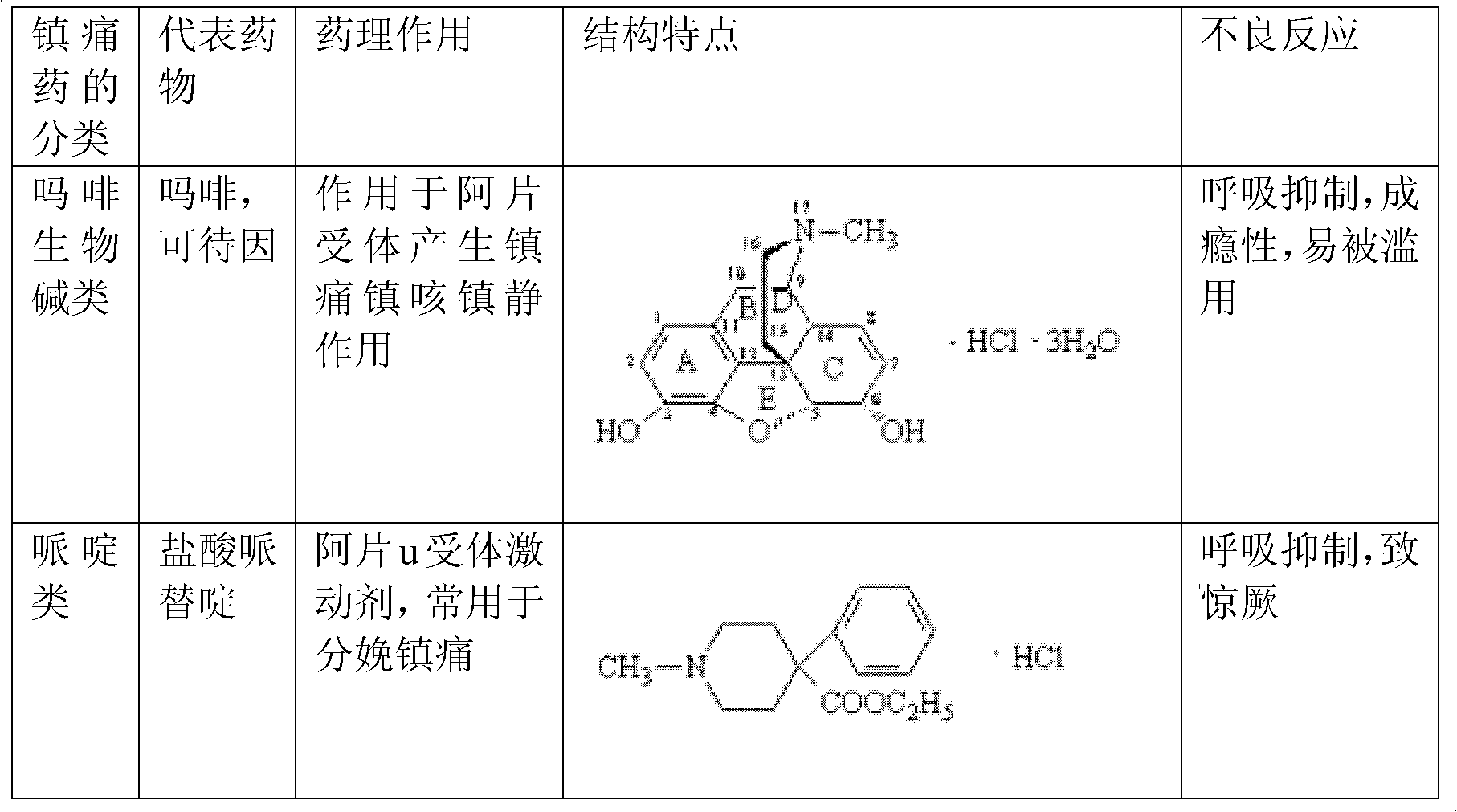
Medical new use of ursolic acid
A new use technology of ursolic acid, which is applied in the field of new medical uses of ursolic acid, can solve the problems of the use of analgesic drugs that have no public records in the literature, and achieve the effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: Ursolic acid hot plate analgesic experiment
[0018] Materials and methods
[0019] Reagents: ursolic acid nano-liposome (90.6%, provided by Wuhan Lyric Pharmaceutical Technology Co., Ltd.), compound ammonium barbiturate injection (Tianjin Jinyao Amino Acid Co., Ltd., approved by the Chinese Medicine H12021217), 500mL physiological salt Aqueous solution (China Otsuka Pharmaceutical Co., Ltd., Zhunzi H1202006), picric acid, absolute ethanol (analytical grade, Tianjin Binhai Sidi Chemical Reagent Co., Ltd.). Take the ursolic acid nanoliposome freeze-dried powder injection, add an appropriate amount of water for injection into each bottle, until completely dissolved, and set aside.
[0020] Instruments: stopwatch, 501 super thermostat (Shanghai No. 2 Hardware Factory), hot plate apparatus, transparent glass cover, 1mL syringe (Shanghai Mishawa Medical Industry Co., Ltd.), 1μL syringe, LD electronic balance (Shenyang Longteng Electronics Co., Ltd.), quartz d...
Embodiment 2
[0033] Embodiment 2: ursolic acid analgesic acetic acid writhing experiment
[0034] Materials and methods
[0035] Reagents: ursolic acid nanoliposome (90.6%), compound ammonium barbiturate injection (Tianjin Jinyao Amino Acid Co., Ltd., approved by the Chinese Medicine H12021217), 1% glacial acetic acid (self-made, 99% glacial acetic acid added to distilled water Prepared), 500mL normal saline solution (China Otsuka Pharmaceutical Co., Ltd., Zhunzi H1202006), picric acid, triple distilled water (self-made), absolute ethanol (analytical grade, Tianjin Binhai Private Chemical Reagent Co., Ltd.). Take the ursolic acid nanoliposome freeze-dried powder injection, add an appropriate amount of water for injection into each bottle, until completely dissolved, and set aside.
[0036] Test drug method: preparation method: take ursolic acid (purity 99.7%, batch number 20090906, provided by Wuhan Lyric Robot Pharmaceutical Technology Co., Ltd.) and add Tween-80 in an appropriate amount...
Embodiment 3
[0048] Embodiment 3: ursolic acid formalin experiment
[0049] material method
[0050] Test drug method: preparation method: take ursolic acid (purity 99.7%, batch number 20090906, provided by Wuhan Lyric Robot Pharmaceutical Technology Co., Ltd.) and add Tween-80 in an appropriate amount, heat in a water bath at 80°C until completely dissolved, add water to dilute, Be made into the solution (UA crude drug) containing ursolic acid 3mg / ml for subsequent use.
[0051] Experimental reagents: ursolic acid nanoliposomes (90.6%), compound ammonium barbiturate injection (Tianjin Jinyao Amino Acid Co., Ltd., national drug approval H12021217), 1500mL physiological sodium chloride solution (China Otsuka Pharmaceutical Co., Ltd. , National Pharmaceutical Standard H1202006), picric acid, triple distilled water (self-made), absolute ethanol (analytical grade, Tianjin Binhai Private Chemical Reagent Co., Ltd.), formalin Animals and groups: 60 healthy adult female mice , weight 18-22g, go...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com