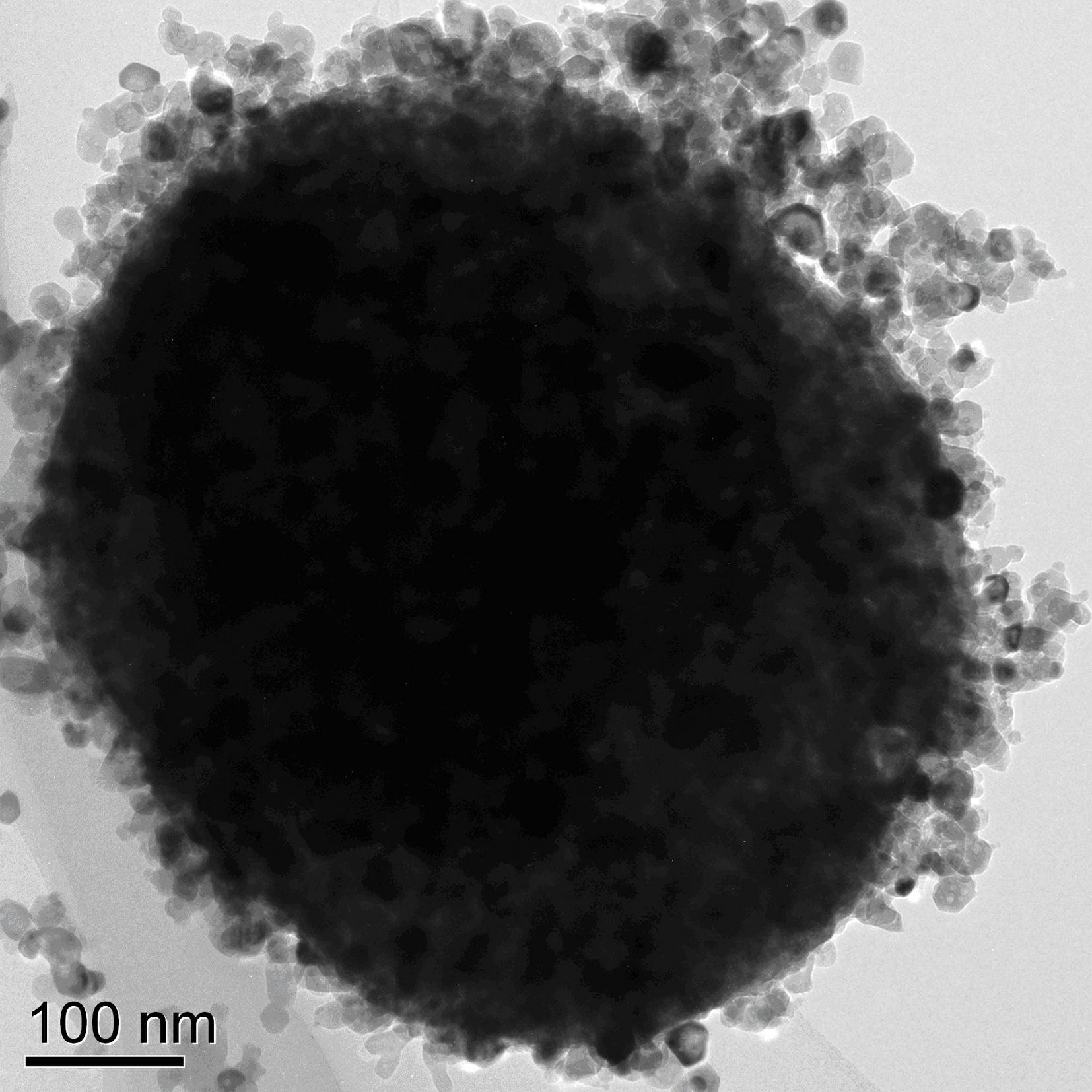
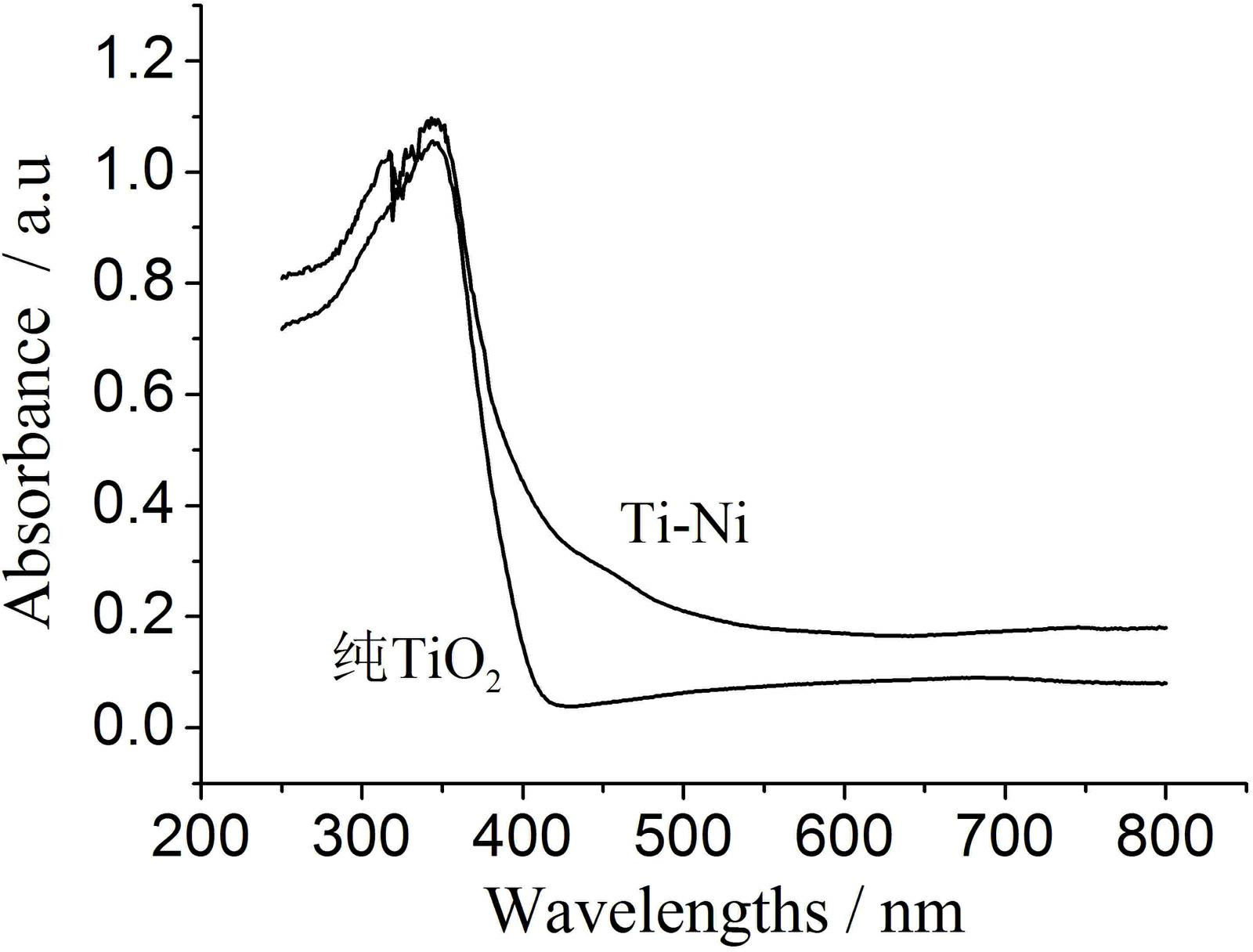
Method for preparing visible light responding spherical titanium dioxide composite photocatalyst with nickel oxide supported on surface
A titanium dioxide, surface-loaded technology, applied in the field of photocatalyst preparation, can solve the problems affecting the photocatalytic efficiency of semiconductors, the photocatalytic efficiency is not very high, the utilization rate of solar energy is low, etc., and achieves low cost, high preparation efficiency and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Step 1: Prepare a lithium chloride solution with a molar concentration of 0.1M; mix the three according to the volume ratio of salt solution: tetrabutyl titanate: absolute ethanol = 0.3: 1.5: 100, and stir vigorously until a titanium dioxide sol is formed; After standing for 24 hours, centrifuge and wash with absolute ethanol, and dry at 80°C for 12 hours under vacuum to obtain spherical titanium dioxide particles;
[0021] Step 2: prepare a nickel chloride solution with a molar concentration of 0.01M; disperse the titanium dioxide microspheres prepared in step 1 into the nickel salt solution in a ratio of 0.001:1 by nickel element and titanium dioxide mass ratio; or according to the mass ratio of nickel: Titanium dioxide: deionized water = 0.001:1:20, first dissolve the nickel salt solid powder with deionized water, then add the titanium dioxide powder prepared in step 1; magnetically stir for 15 minutes and then ultrasonically disperse for 15 minutes, so that the titan...
Embodiment 2
[0023] Step 1: Prepare a sodium chloride solution with a molar concentration of 0.1M; mix the three according to the volume ratio of salt solution: tetrabutyl titanate: absolute ethanol = 0.4:2:100, and stir vigorously until a titanium dioxide sol is formed; After standing for 24 hours, centrifuge and wash with absolute ethanol, and dry at 80°C for 12 hours under vacuum to obtain spherical titanium dioxide particles;
[0024] Step 2: prepare a nickel nitrate solution with a molar concentration of 0.05M; disperse the titanium dioxide microspheres prepared in step 1 into the nickel salt solution at a ratio of 0.01:1 by mass ratio of nickel element to titanium dioxide; or according to the mass ratio of nickel:titanium dioxide : deionized water=0.01:1:20, first dissolve the nickel salt solid powder with deionized water, then add the titanium dioxide powder prepared in step 1; magnetically stir for 15 minutes and then ultrasonically disperse for 15 minutes to completely disperse the...
Embodiment 3
[0026] Step 1: Prepare a sodium chloride solution with a molar concentration of 0.1M; mix the three according to the volume ratio of salt solution: tetrabutyl titanate: absolute ethanol = 0.5:2:100, and stir vigorously until a titanium dioxide sol is formed; After standing for 24 hours, centrifuge and wash with absolute ethanol, and dry at 80°C for 12 hours under vacuum to obtain spherical titanium dioxide particles;
[0027] Step 2: Prepare a nickel sulfate solution with a molar concentration of 0.05M; disperse the titanium dioxide microspheres prepared in step 1 into the nickel salt solution at a ratio of 0.01:1 by mass ratio of nickel element to titanium dioxide; or according to the mass ratio nickel:titanium dioxide : deionized water=0.01:1:20, first dissolve the nickel salt solid powder with deionized water, then add the titanium dioxide powder prepared in step 1; magnetically stir for 15 minutes and then ultrasonically disperse for 15 minutes to completely disperse the ti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com