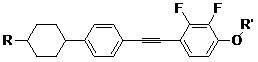
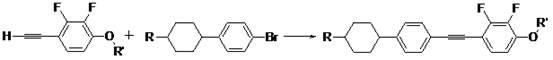1-alkoxy-2, 3-difluoro-4-(2-(4-(trans-4-alkyl cyclohexyl) phenyl) acetenyl) benzene and preparation method thereof
A technology of alkoxybenzene and alkoxy, which is applied in the field of benzyne-based negative liquid crystal materials, can solve the problems of poor chemical stability and photochemical stability, weak negative dielectric anisotropy, and high viscosity of liquid crystal materials. Good stability and photochemical stability, enhanced negative dielectric anisotropy, and moderate negative dielectric anisotropy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Under the protection of nitrogen, 0.1725mol, 46.06gg (1.0eq) of 4-(4-ethylcyclohexyl)bromobenzene, 0.1725mol, 28.98g (1.0eq) 2,3-difluoro-4-methyl Oxyphenyl acetylene, 4.0g palladium-carbon, 0.1725mol, 0.328g (0.01eq) cuprous iodide, 0.00345mol, 0.904g triphenylphosphine, 0.1725mol, 18.3g sodium carbonate, 200ml, 1.9mol toluene into the reaction flask In the medium, start stirring, heat up to 50°C to keep the temperature for the reaction, and control the gas phase. After the reaction of the raw materials is completed, the palladium-carbon is removed by suction filtration, the reaction solution is frozen until the product precipitates, and the 1-methoxy-2,3-bis is obtained after suction filtration Crude fluoro-4-[2-[4-(trans-4-ethylcyclohexyl)phenyl]ethynyl]benzene. To the above crude 1-methoxy-2,3-difluoro-4-[2-[4-(trans-4-ethylcyclohexyl)phenyl]ethynyl]benzene was added 5 times equivalent of ethanol and 5% equivalent of activated carbon is heated and refluxed for re...
Embodiment 2
[0024] Under the protection of nitrogen, 0.1725mol, 48.47g (1.0eq) of 4-(4-propylcyclohexyl) bromobenzene, 0.19mol, 34.5g (1.1eq) 2,3-difluoro-4-ethane Oxyphenyl acetylene, 4.0g palladium on carbon, 0.1725mol, 0.328g (0.01eq) cuprous iodide, 0.00345mol, 0.904g triphenylphosphine, 0.1725mol, 18.3g sodium carbonate, 200ml, 1.4mol triethylamine In the reaction flask, turn on the stirring, heat up to 60°C to keep the reaction warm, and control the gas phase. After the reaction of the raw materials is completed, the palladium carbon is removed by suction filtration. The reaction solution is frozen until the product precipitates. After suction filtration, 1-ethoxy-2,3 is obtained. -Crude difluoro-4-[2-[4-(trans-4-propylcyclohexyl)phenyl]ethynyl]benzene. To the crude 1-ethoxy-2,3-difluoro-4-[2-[4-(trans-4-propylcyclohexyl)phenyl]ethynyl]benzene was added 5 times equivalent of petroleum ether and 5% equivalent of activated carbon is heated and refluxed to recrystallize. The activat...
Embodiment 3
[0026] Under the protection of nitrogen, 0.1725mol, 54.34g (1.0eq) of 4-(4-pentylcyclohexyl) bromobenzene, 0.207mol, 46.37g (1.2eq) 2,3-difluoro-4-pentane Oxyphenyl acetylene, 4.0g palladium-carbon, 0.1725mol, 0.328g (0.01eq) cuprous iodide, 0.00345mol, 0.904g triphenylphosphine, 0.1725mol, 18.3g sodium carbonate, 200ml, 1.9mol toluene into the reaction flask In the medium, turn on the stirring, heat up to 80°C to keep the reaction, and control the gas phase. After the raw material reaction is completed, the palladium-carbon is removed by suction filtration, the reaction solution is frozen until the product precipitates, and the 1-pentyloxy-2,3-bis is obtained after suction filtration Crude fluoro-4-[2-[4-(trans-4-pentylcyclohexyl)phenyl]ethynyl]benzene. Add 5 times equivalent of petroleum ether to the above crude 1-pentoxy-2,3-difluoro-4-[2-[4-(trans-4-pentylcyclohexyl)phenyl]ethynyl]benzene And 5% equivalent of activated carbon heated and refluxed for recrystallization, suct...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com