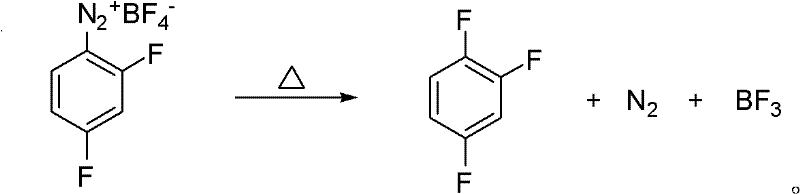
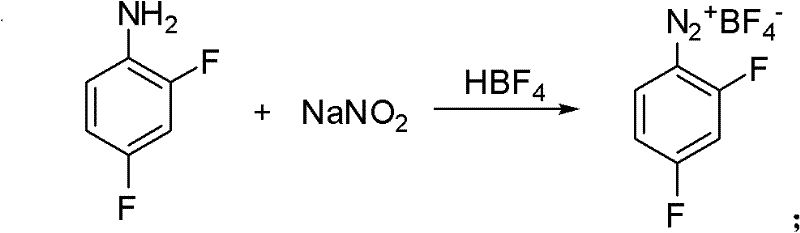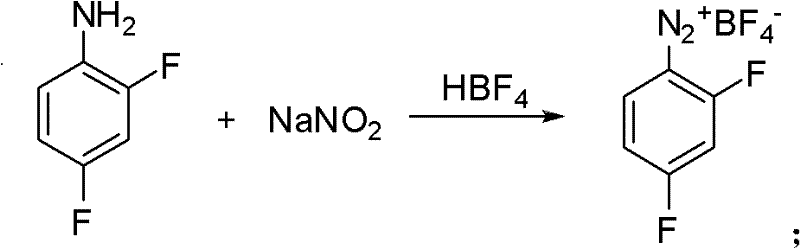Preparation method of 1,2,4-trifluorobenzene
A technology for trifluorobenzene and difluoroaniline, which is applied in the field of chemical drug preparation, can solve the problems of harsh operating conditions, high production cost, expensive raw materials, etc., and achieves the effects of low production cost, easy operation and low requirement for reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Preparation of diazonium fluoroborate.
[0027] Add 1760g 30% fluoroboric acid aqueous solution (fluoroboric acid content: 6mol) in a 3000ml four-neck flask, add 258g (2mol) 2,4-difluoroaniline dropwise at room temperature, the reaction is exothermic, continue to stir at room temperature for 1 hour after dropping, and then lower the temperature To -5 ~ 0 ℃. Add dropwise a solution made of 145g (2.1mol) sodium nitrite and 340g water, exothermic reaction, control the rate of addition, keep the reaction temperature not exceeding 5°C, continue the reaction at 0-5°C for 2 hours after the addition, filter, The solid was washed successively with 300ml of water precooled to 0°C and 200ml of ethanol, and after drying, 410g of diazonium fluoroborate was obtained with a yield of 90%.
Embodiment 2
[0028] Embodiment 2: Preparation of diazonium fluoroborate.
[0029] Add 1760g 20% fluoroboric acid aqueous solution (fluoroboric acid content: 4mol) in a 3000ml four-neck flask, add 258g (2mol) 2,4-difluoroaniline dropwise at room temperature, the reaction is exothermic, continue to stir at room temperature for 1 hour after dropping, and then lower the temperature To -5 ~ 0 ℃. Add dropwise a solution made of 141g (2.04mol) sodium nitrite and 340g water, exothermic reaction, control the rate of addition, keep the reaction temperature not exceeding 5°C, and continue the reaction at -10--5°C for 4 hours after the addition is complete. After filtering, the solid was washed successively with 300ml of water and 200ml of ethanol precooled to 0°C, and after drying, 415g of diazonium fluoroborate was obtained, with a yield of 91%.
Embodiment 3
[0030] Embodiment 3: Preparation of diazonium fluoroborate.
[0031] Add 2200g 40% fluoboric acid solution (fluoboric acid content: 10mol) in a 3000ml four-neck flask, add 258g (2mol) 2,4-difluoroaniline dropwise at room temperature, the reaction is exothermic, continue to stir at room temperature for 1 hour after dropping, and then lower the temperature To -5 ~ 0 ℃. Add dropwise a solution made of 152g (2.2mol) sodium nitrite and 360g water, exothermic reaction, control the rate of addition, keep the reaction temperature not exceeding 5°C, and continue the reaction at -20~-10°C for 6 hours after the addition is complete. After filtering, the solid was washed successively with 300ml of water and 200ml of ethanol precooled to 0°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com