Poly alkyl ether compound with strange end group and double functional groups and application thereof
A technology of hetero-difunctional compound, which is applied in the field of hetero-difunctional polyalkyl ether compounds, can solve the problem of non-reaction specificity of succinimide ester groups, non-repeatability of coupling products, and chemical coupling process. Poor controllability and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Example 1 Preparation of thiobenzenesulfonate-PEG-hydrazide (~2000Da)
[0091] 350mg of polyethylene glycol (average molecular weight 2000Da) with a hydroxyl group at one end and a carboxyl group at the other end, with a chemical structure of the formula (i-A1):
[0092]
[0093] Among them, p=2, n=2, m=0, n=0, w=1, X=-O-, Y=-O-, PAG=-(CH 2 CH 2 O) h - (the average molecular weight of PAG is around 2000Da), that is, it is expressed as the following structure:
[0094]
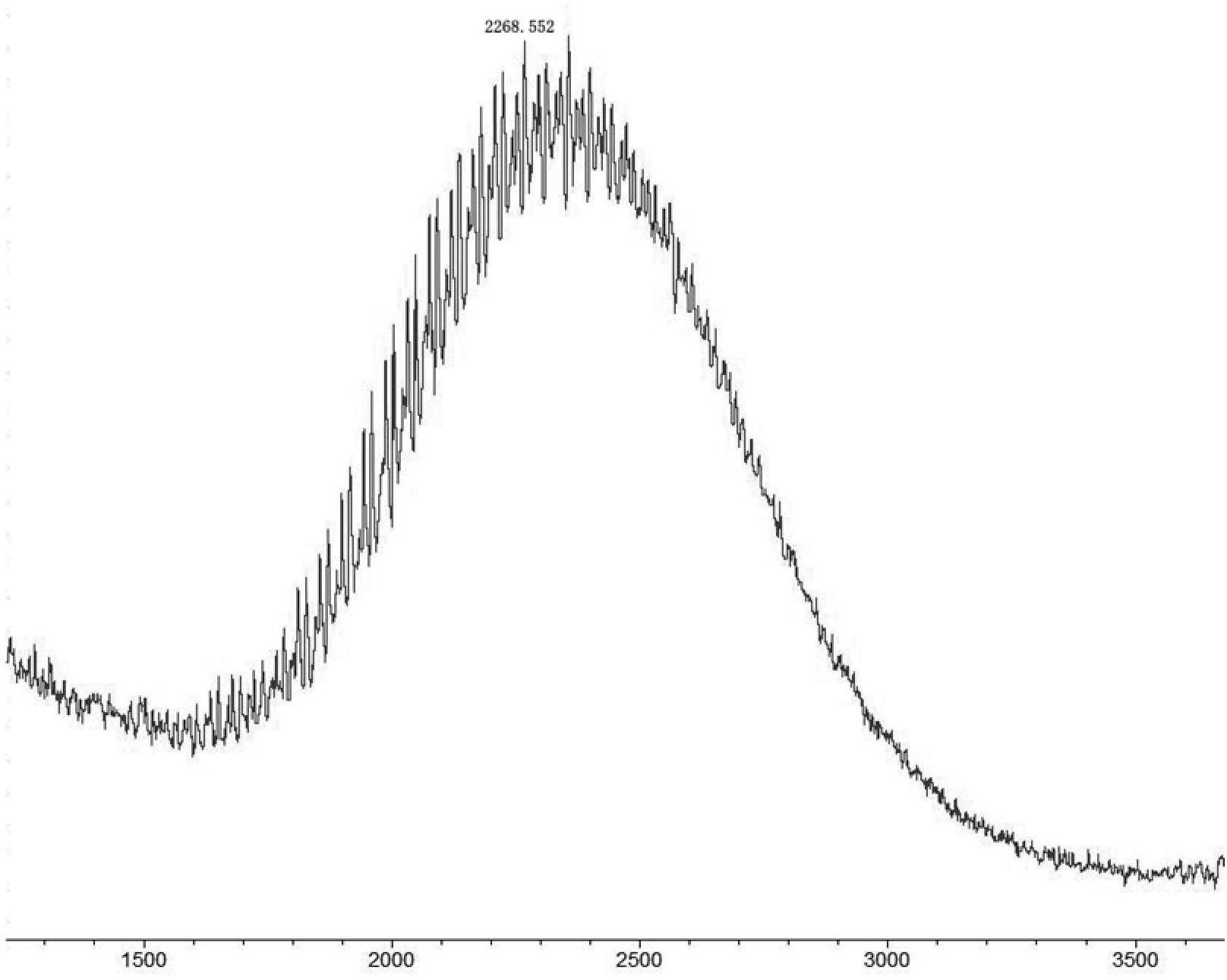
[0095] Azeotropic removal of water with toluene, based on polyethylene glycol with one terminal hydroxyl and one terminal carboxyl group, sequentially add 1.5 times the molar amount of phosphorus tribromide and 2.5 times the molar amount of triethylamine, react at 60°C for 2 days, and then evaporate toluene to dryness , re-dissolved in brine, extracted with dichloromethane, dried over anhydrous sodium sulfate and filtered, the filtrate was concentrated, precipitated with ether, filtered, and a w...
Embodiment 2
[0107] Example 2 Preparation of thiobenzenesulfonate-PEG-hydrazide (~200Da)
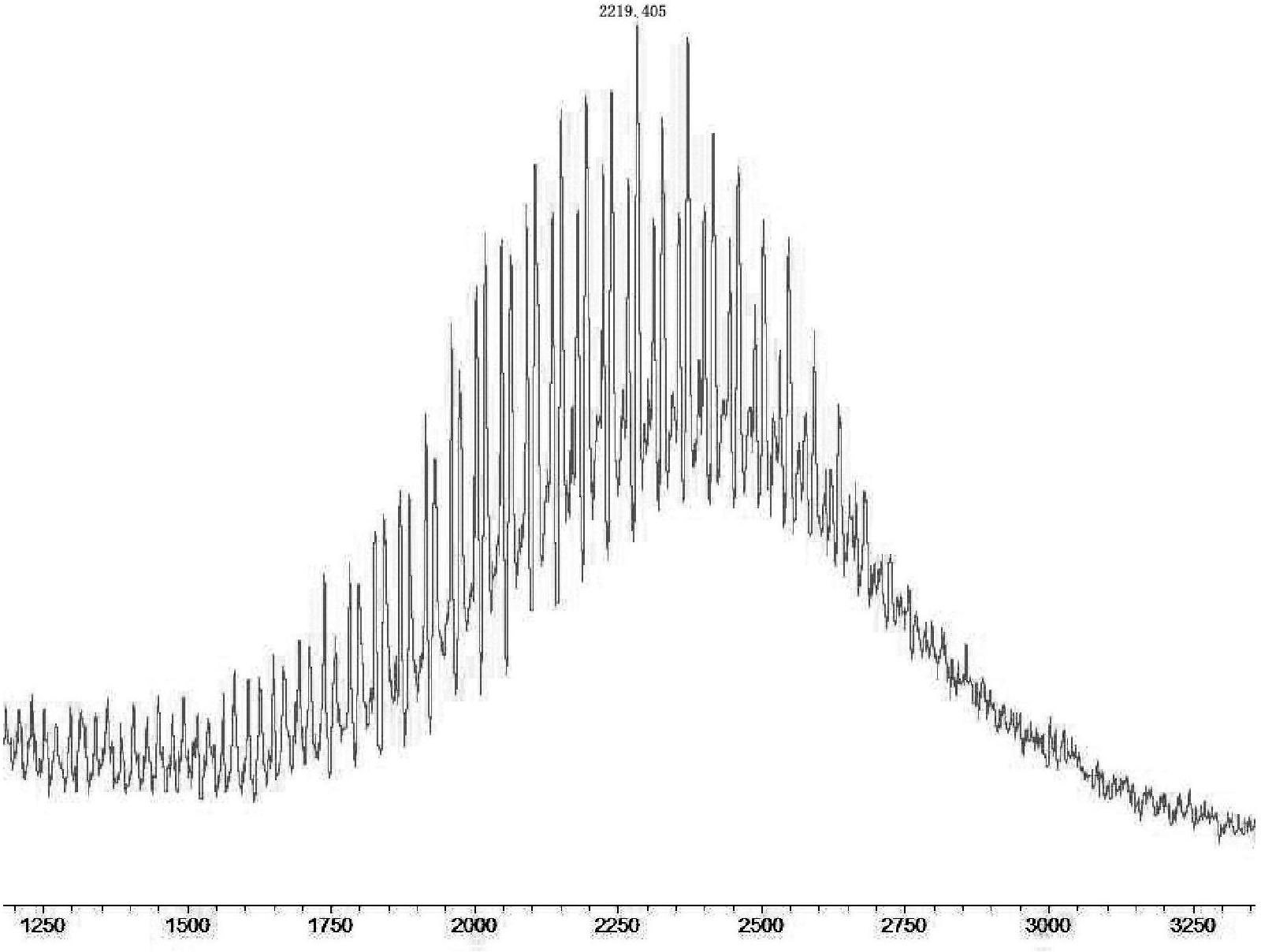
[0108] 200 mg of polyethylene glycol (molecular weight: 164 Da) with a hydroxyl group at one end and a carboxyl group at the end, having the chemical structure of formula (i-A1) described in Example 1, wherein p=4, n=1, m=4, n=1, w=2, X=-O-, Y=-O-, PAG=-(CH 2 CH 2 O) h - (wherein the average molecular weight of PAG is about 200Da), azeotrope with toluene to remove water, and then add 1.5 times the molar amount of phosphorus tribromide and 2.5 times the molar amount of three Ethylamine, reacted at 60°C for 1 day, then evaporated toluene to dryness, redissolved in brine, extracted with dichloromethane, dried over anhydrous sodium sulfate, filtered, concentrated the filtrate, and evaporated to dryness to obtain a light yellow viscous liquid, which was detected by mass spectrometry as 227.1, confirmed It is polyethylene glycol with bromine at one end and carboxyl at the other end.
[0109] Use toluen...
Embodiment 3
[0112] The preparation of embodiment 3 thiobenzenesulfonate-PEG-oxoamine
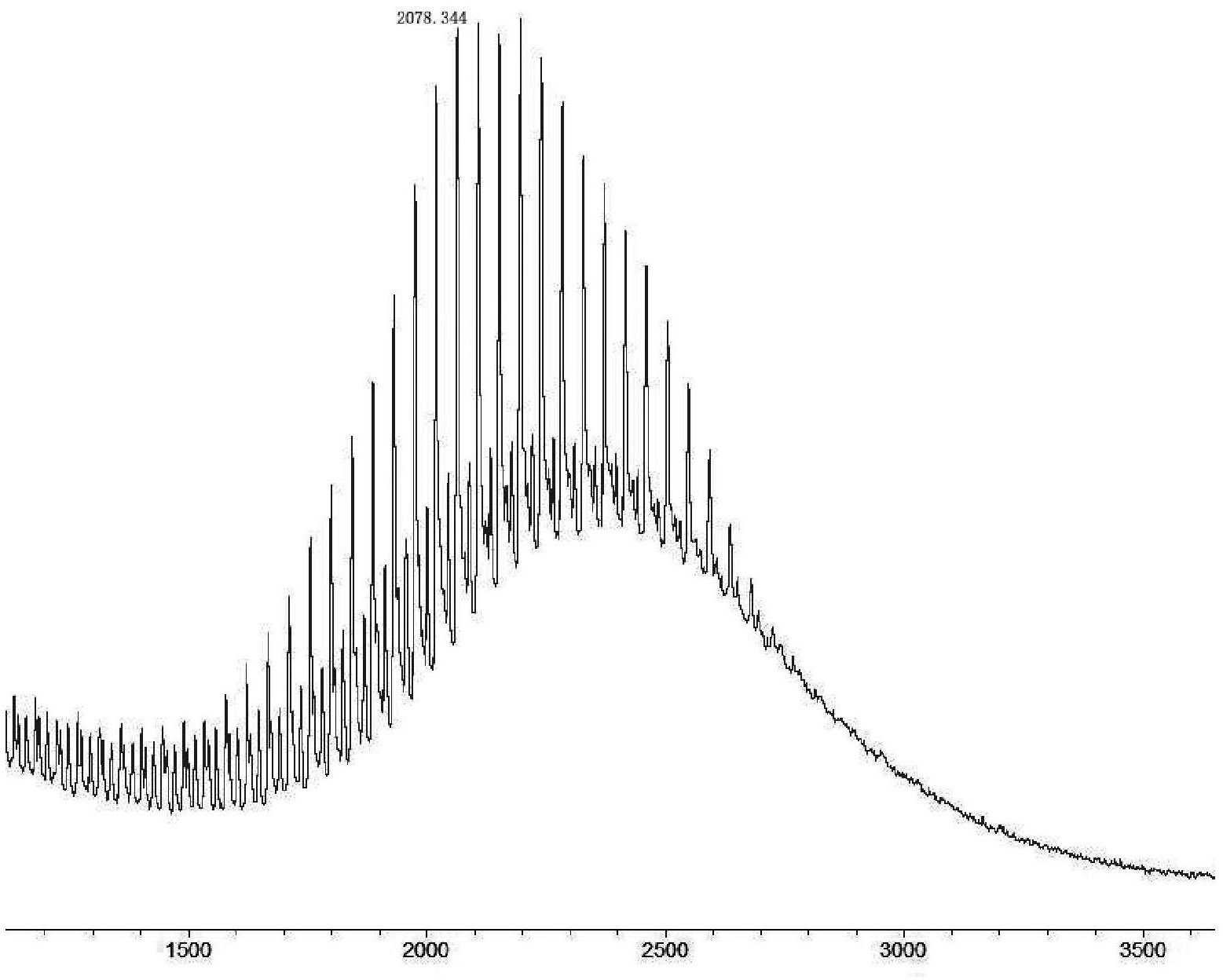
[0113]500 mg of polyethylene glycol (average molecular weight 2000 Da) with a hydroxyl group at one end and bromine at one end was dissolved in DMF, and 1.5 times the molar amount of N-hydroxyphthalimide and 1.5 times the molar amount of Triethylamine, reacted at 80°C for 1 day, evaporated to dryness, redissolved with brine, filtered, extracted the filtrate with dichloromethane, dried with anhydrous sodium sulfate, filtered, concentrated the filtrate, precipitated with ether, filtered to obtain a white solid powder, mass spectrometry It was detected as 2102.344±44.052n, and it was confirmed to be polyethylene glycol with a hydroxyl group at one end and a phthalimide at the other end.
[0114] Use toluene to remove water azeotropically from the polyethylene glycol with one hydroxyl at one end and phthalimide at one end obtained in the previous step, and then add 1.5 times the molar amount of tribromo Ph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Average molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com