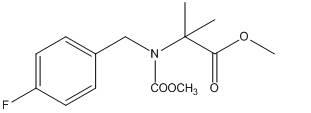
Synthetic method for 2-(N-4-fluorobenzyl) methoxy-acetyl-amino methyl isobutyrate
A technology of methyl methoxyacetamidoisobutyrate and methyl fluorobenzylidene aminoacetate, which is applied in the field of organic chemical synthesis, can solve the problems of difficult electrocarboxylation, complicated preparation process, and poor stability, and achieves The effects of reducing air pollution, cheap and easy-to-obtain raw materials, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] a. Preparation of electrolyte
[0017] Mix 0.001mol (0.195g) methyl 4-fluorobenzylidene aminoacetate with 0.001mol (0.210g) tetraethylammonium bromide and 0.129mol (10 mL) N,N-dimethylformamide to form an electrolyte, Then put it into a one-compartment electrolytic cell with stainless steel as the cathode and magnesium rod as the anode, methyl 4-fluorobenzylidene aminoacetate, tetraethylammonium bromide and N,N-dimethylformamide are analytically pure, of which : Methyl 4-fluorobenzylidene aminoacetate is the substrate, N,N-dimethylformamide is the solvent after drying with 4? grade molecular sieves, and tetraethylammonium bromide is the supporting electrolyte.
[0018] b. Electrocarboxylation reaction
[0019] Under normal pressure, carbon dioxide is introduced into the electrolytic cell to saturation, and then at 2.9mA / cm 2 The electrocarboxylation reaction was carried out at a constant current density with a flow rate of 193C and a reaction temperature of 25°C.
[...
Embodiment 2
[0025] a. Preparation of electrolyte
[0026] Mix 0.001mol (0.195g) methyl 4-fluorobenzylidene aminoacetate with 0.001mol (0.257g) tetraethylammonium iodide and 0.129mol (10 mL) N,N-dimethylformamide to form an electrolyte, Then put it into a one-chamber electrolytic cell with stainless steel as the cathode and magnesium rod as the anode, methyl 4-fluorobenzylidene aminoacetate, tetraethylammonium iodide and N,N-dimethylformamide are analytically pure, of which : Methyl 4-fluorobenzylidene aminoacetate is the substrate, N,N-dimethylformamide is the solvent after drying with 4? grade molecular sieves, and tetraethylammonium iodide is the supporting electrolyte.
[0027] b. Electrocarboxylation reaction
[0028] Under normal pressure, carbon dioxide is introduced into the electrolytic cell to saturation, and then at 2.9mA / cm 2 The electrocarboxylation reaction was carried out at a constant current density with a flow rate of 193C and a reaction temperature of 25°C.
[0029] c...
Embodiment 3
[0034] a. Preparation of electrolyte
[0035] Mix 0.001mol (0.195g) methyl 4-fluorobenzylidene aminoacetate with 0.001mol (0.165g) tetraethylammonium chloride and 0.129mol (10 mL) N,N-dimethylformamide to form an electrolyte, Then put it into a one-chamber electrolytic cell with stainless steel as the cathode and magnesium rod as the anode, methyl 4-fluorobenzylidene aminoacetate, tetraethylammonium chloride and N,N-dimethylformamide are analytically pure, of which : Methyl 4-fluorobenzylidene aminoacetate is the substrate, N,N-dimethylformamide is the solvent after drying with 4? grade molecular sieves, and tetraethylammonium chloride is the supporting electrolyte.
[0036] b. Electrocarboxylation reaction
[0037] Under normal pressure, carbon dioxide is introduced into the electrolytic cell to saturation, and then at 2.9mA / cm 2 The electrocarboxylation reaction was carried out at a constant current density with a flow rate of 193C and a reaction temperature of 22°C.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com