Method for preparing chalcopyrite structure CuInSe2 or/and CuInSe2/ZnS core-shell structure quantum dots
A core-shell structure and quantum dot technology, which is applied in the field of compound semiconductor nanomaterial preparation, can solve the problems of quantum dot application limitation, expensive raw material price, and fluorescence quantum yield less than 5%, avoiding the use of precursor raw materials and simplifying the preparation. Process and the effect of mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1) Weigh 29.2mg (0.1mmol) In(Ac) 3 , 9.9mg (0.1mmol) CuCl in a 25ml four-neck flask, and add 1.39ml (8mmol) n-octyl mercaptan, 6ml of octadecene, magnetic stirring for 30min under vacuum conditions, until the cationic precursor liquid is completely dissolved to To clarify, get Cu + 、In 3+ Cation precursor solution: Weigh 31.6mg (0.4mmol) of elemental Se in a single-mouth round bottom bottle, add 536ul (1.2mmol) of TOP, 2ml of octadecene, dissolve until clear to obtain Se precursor solution.
[0040] 2) Backfill the system with argon, and the Cu + 、In 3+ The cation precursor solution was rapidly heated from room temperature to 230°C, injected into the Se precursor solution, reacted for 45 minutes, removed the heat source, cooled to room temperature, and centrifuged to obtain CuInSe 2 quantum dots;
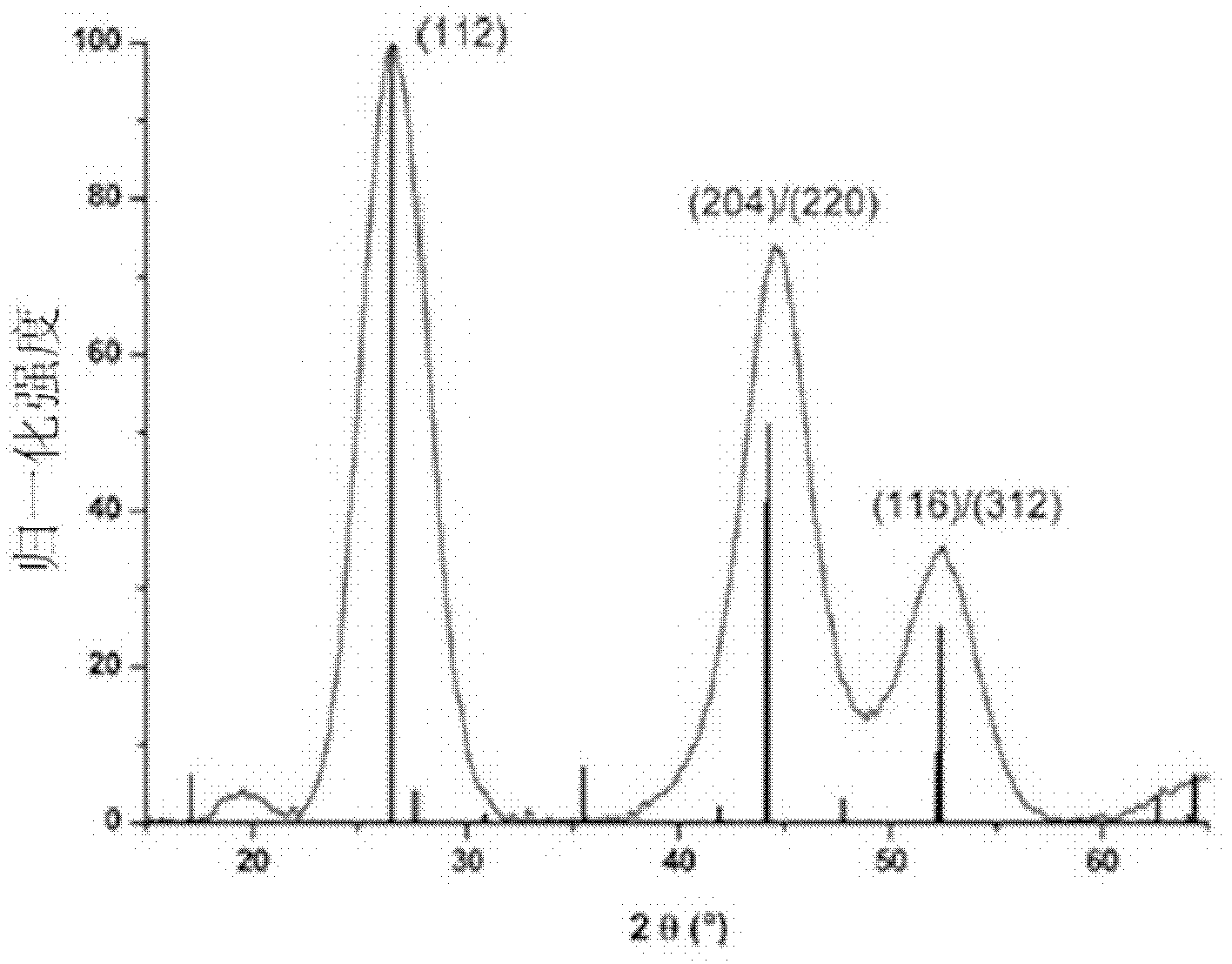
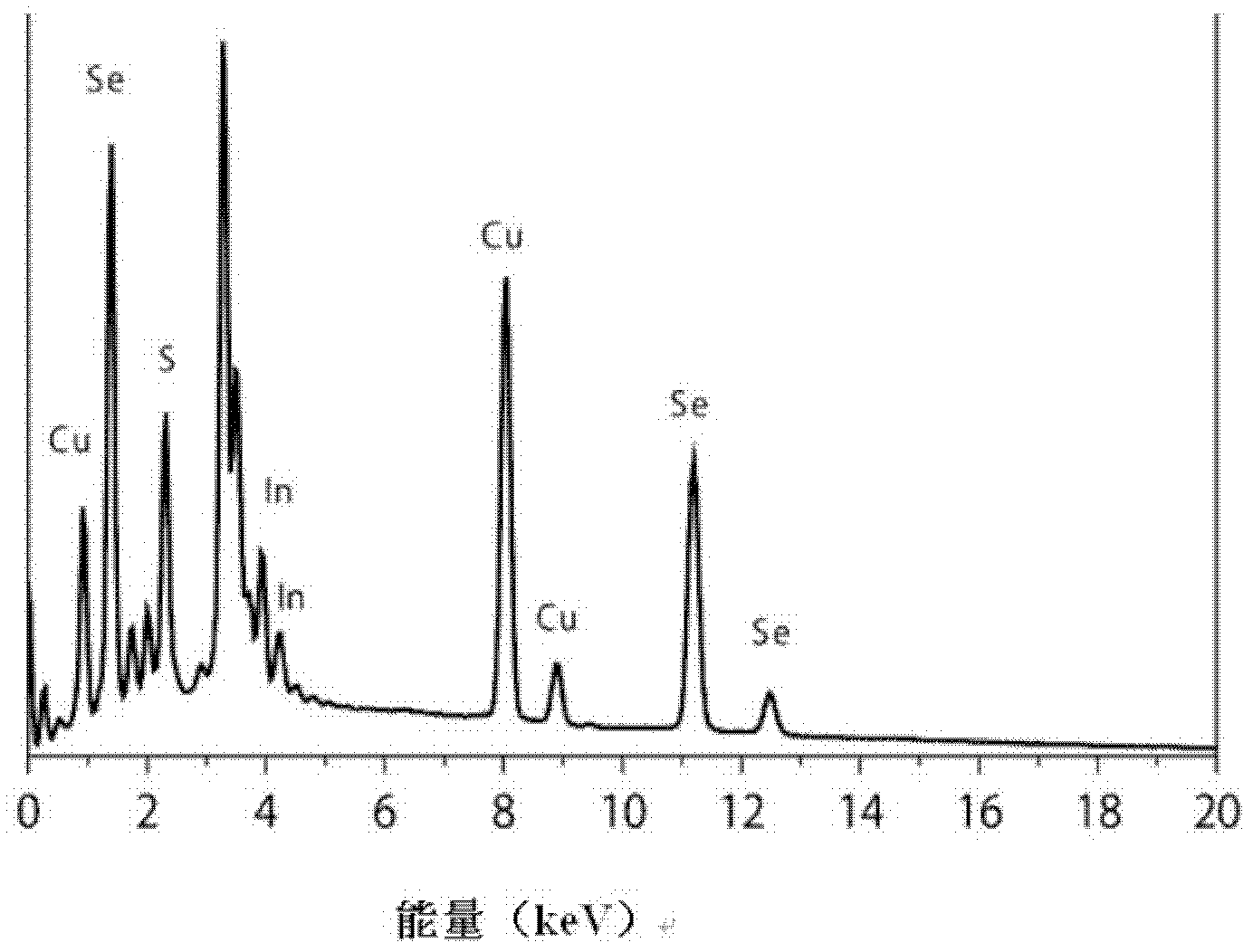
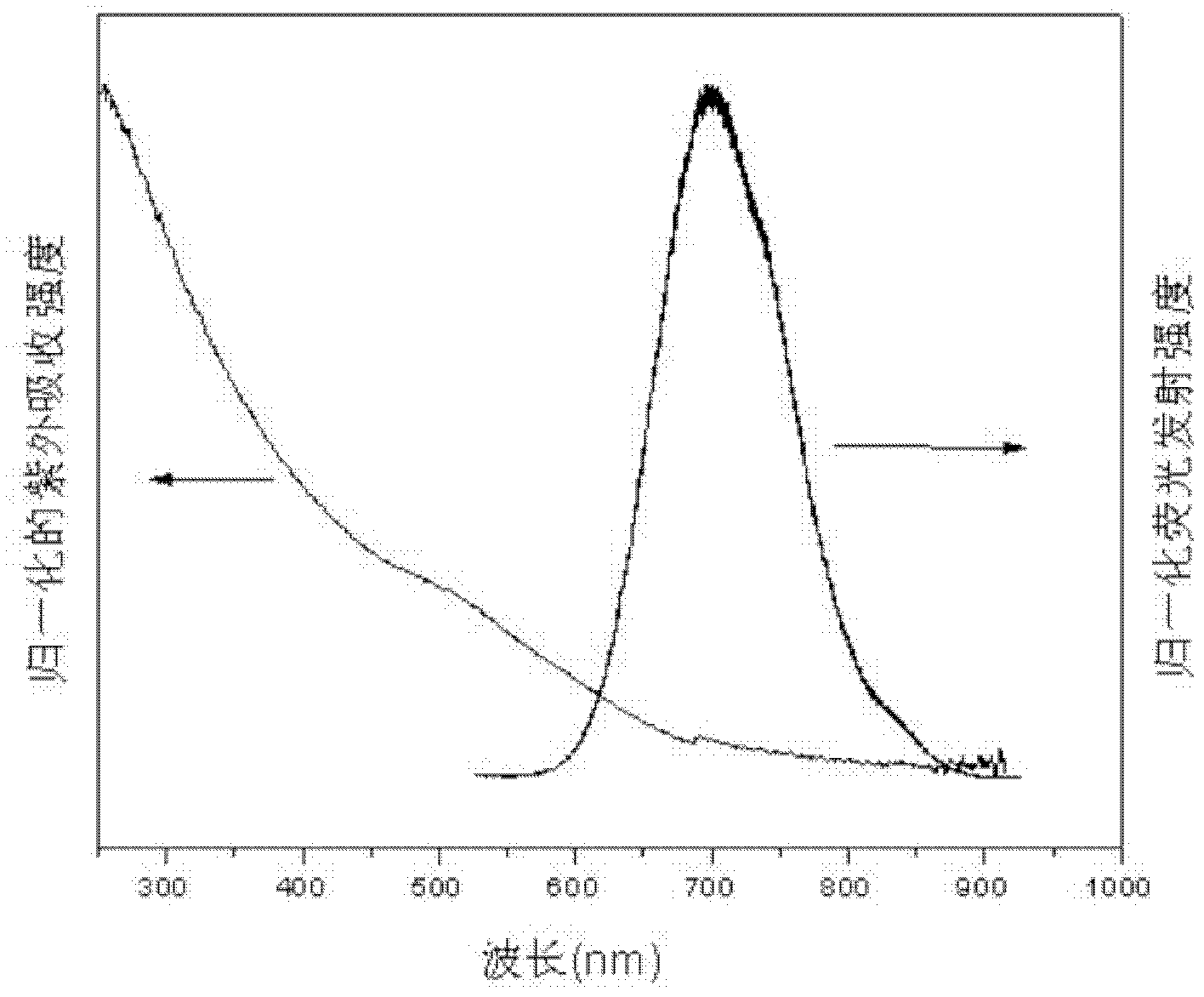
[0041] Such as figure 1 As shown, the obtained CuInSe 2 The XRD spectrum of quantum dots proves that the obtained nanocrystals are chalcopyrite structures; as figure...
Embodiment 2
[0043] 1) Weigh 44.2mg (0.2mmol) In(Cl) 3 , 19.1mg (0.1mmol) CuI in a 25ml four-neck flask, and add 1.43ml (6mmol) dodecyl mercaptan, 8ml of octadecene, magnetic stirring for 30min under vacuum conditions, until the cationic precursor liquid is completely Dissolve until clear to give Cu + 、In 3+ Cation precursor solution: Weigh 47.4mg (0.6mmol) of elemental Se into a single-mouth round bottom bottle, add 450ul (1.8mmol) of TBP and 3ml of octadecene, dissolve until clear to obtain Se precursor solution.
[0044] 2) Backfill the system with argon, and the Cu + 、In 3+ The cation precursor solution was rapidly heated from room temperature to 200°C, injected into the Se precursor solution, reacted for 15 minutes, removed the heat source, cooled to room temperature, and centrifuged to obtain CuInSe 2 quantum dots;
[0045] Such as figure 1 As shown, the obtained CuInSe 2 The XRD spectrum of quantum dots proves that the obtained nanocrystals are chalcopyrite structures; as f...
Embodiment 3
[0047] 1) Weigh 148.7mg (0.3mmol) InI 3 , 12.3mg (0.1mmol) CuAc in a 25ml four-neck flask, and add 1.35ml (4mmol) octadecyl mercaptan, 7ml of octadecene, magnetic stirring under vacuum conditions for 30min, until the cationic precursor liquid is completely Dissolve until clear to give Cu + 、In 3+ Cation precursor solution: Weigh 632mg (0.8mmol) of elemental Se in a single-mouth round bottom bottle, add 600ul (2.4mmol) of TBP and 4ml of octadecene, and dissolve until clarified to obtain Se precursor solution.
[0048] 2) Backfill the system with argon, and the Cu + 、In 3+ The cation precursor solution was rapidly heated from room temperature to 250 °C, injected into the Se precursor solution, reacted for 60 minutes, removed the heat source and cooled to room temperature, and centrifuged to obtain CuInSe 2 quantum dots;
[0049] Such as figure 1 As shown, the obtained CuInSe 2 The XRD spectrum of quantum dots proves that the obtained nanocrystals are chalcopyrite structur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com